J Bone Metab.
2017 Aug;24(3):147-153. 10.11005/jbm.2017.24.3.147.
Regulation of Cartilage Development and Diseases by Transcription Factors
- Affiliations
-
- 1Department of Molecular and Cellular Biochemistry, Osaka University Graduate School of Dentistry, Osaka, Japan. rikonisi@dent.osaka-u.ac.jp
- KMID: 2415952
- DOI: http://doi.org/10.11005/jbm.2017.24.3.147
Abstract
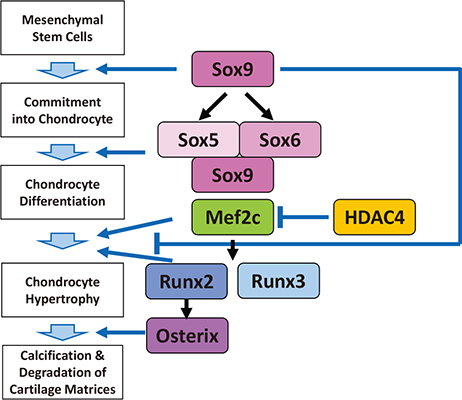
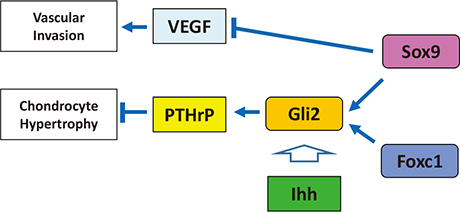
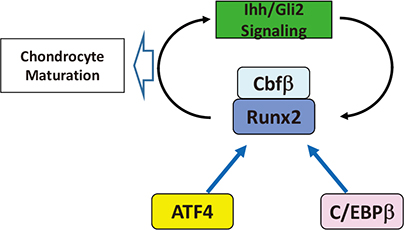
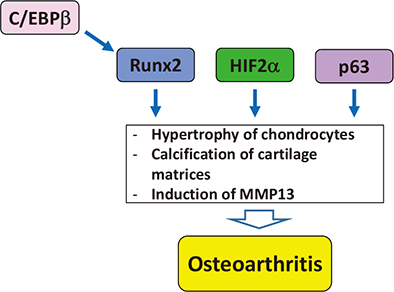
- Genetic studies and molecular cloning approaches have been successfully used to identify several transcription factors that regulate the numerous stages of cartilage development. Sex-determining region Y (SRY)-box 9 (Sox9) is an essential transcription factor for the initial stage of cartilage development. Sox5 and Sox6 play an important role in the chondrogenic action of Sox9, presumably by defining its cartilage specificity. Several transcription factors have been identified as transcriptional partners for Sox9 during cartilage development. Runt-related transcription factor 2 (Runx2) and Runx3 are necessary for hypertrophy of chondrocytes. CCAAT/enhancer-binding protein β (C/EBPβ) and activating transcription factor 4 (ATF4) function as co-activators for Runx2 during hypertrophy of chondrocytes. In addition, myocyte-enhancer factor 2C (Mef2C) is required for initiation of chondrocyte hypertrophy, presumably by functioning upstream of Runx2. Importantly, the pathogenic roles of several transcription factors in osteoarthritis have been demonstrated based on the similarity of pathological phenomena seen in osteoarthritis with chondrocyte hypertrophy. We discuss the importance of investigating cellular and molecular properties of articular chondrocytes and degradation mechanisms in osteoarthritis, one of the most common cartilage diseases.
Keyword
MeSH Terms
Figure
Reference
-
1. Nishimura R, Hata K, Matsubara T, et al. Regulation of bone and cartilage development by network between BMP signalling and transcription factors. J Biochem. 2012; 151:247–254.
Article2. Hata K, Takahata Y, Murakami T, et al. Transcriptional network controlling endochondral ossification. J Bone Metab. 2017; 24:75–82.
Article3. Akiyama H, Chaboissier MC, Martin JF, et al. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002; 16:2813–2828.
Article4. Foster JW, Dominguez-Steglich MA, Guioli S, et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994; 372:525–530.
Article5. Wagner T, Wirth J, Meyer J, et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994; 79:1111–1120.
Article6. Lefebvre V, Huang W, Harley VR, et al. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol Cell Biol. 1997; 17:2336–2346.
Article7. Takigawa Y, Hata K, Muramatsu S, et al. The transcription factor Znf219 regulates chondrocyte differentiation by assembling a transcription factory with Sox9. J Cell Sci. 2010; 123:3780–3788.
Article8. Smits P, Dy P, Mitra S, et al. Sox5 and Sox6 are needed to develop and maintain source, columnar, and hypertrophic chondrocytes in the cartilage growth plate. J Cell Biol. 2004; 164:747–758.
Article9. Saito T, Ikeda T, Nakamura K, et al. S100A1 and S100B, transcriptional targets of SOX trio, inhibit terminal differentiation of chondrocytes. EMBO Rep. 2007; 8:504–509.
Article10. Hattori T, Muller C, Gebhard S, et al. SOX9 is a major negative regulator of cartilage vascularization, bone marrow formation and endochondral ossification. Development. 2010; 137:901–911.
Article11. Amano K, Hata K, Sugita A, et al. Sox9 family members negatively regulate maturation and calcification of chondrocytes through up-regulation of parathyroid hormone-related protein. Mol Biol Cell. 2009; 20:4541–4551.
Article12. Yoshida M, Hata K, Takashima R, et al. The transcription factor Foxc1 is necessary for Ihh-Gli2-regulated endochondral ossification. Nat Commun. 2015; 6:6653.
Article13. Kawakami Y, Tsuda M, Takahashi S, et al. Transcriptional coactivator PGC-1alpha regulates chondrogenesis via association with Sox9. Proc Natl Acad Sci U S A. 2005; 102:2414–2419.
Article14. Nakamura Y, Yamamoto K, He X, et al. Wwp2 is essential for palatogenesis mediated by the interaction between Sox9 and mediator subunit 25. Nat Commun. 2011; 2:251.
Article15. Zou W, Chen X, Shim JH, et al. The E3 ubiquitin ligase Wwp2 regulates craniofacial development through mono-ubiquitylation of Goosecoid. Nat Cell Biol. 2011; 13:59–65.
Article16. Muramatsu S, Wakabayashi M, Ohno T, et al. Functional gene screening system identified TRPV4 as a regulator of chondrogenic differentiation. J Biol Chem. 2007; 282:32158–32167.
Article17. Amano K, Hata K, Muramatsu S, et al. Arid5a cooperates with Sox9 to stimulate chondrocyte-specific transcription. Mol Biol Cell. 2011; 22:1300–1311.
Article18. Hata K, Nishimura R, Muramatsu S, et al. Paraspeckle protein p54nrb links Sox9-mediated transcription with RNA processing during chondrogenesis in mice. J Clin Invest. 2008; 118:3098–3108.
Article19. Masuda K, Ripley B, Nishimura R, et al. Arid5a controls IL-6 mRNA stability, which contributes to elevation of IL-6 level in vivo. Proc Natl Acad Sci U S A. 2013; 110:9409–9414.
Article20. Hata K, Takashima R, Amano K, et al. Arid5b facilitates chondrogenesis by recruiting the histone demethylase Phf2 to Sox9-regulated genes. Nat Commun. 2013; 4:2850.
Article21. Yoshida CA, Yamamoto H, Fujita T, et al. Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev. 2004; 18:952–963.
Article22. Kobayashi T, Soegiarto DW, Yang Y, et al. Indian hedgehog stimulates periarticular chondrocyte differentiation to regulate growth plate length independently of PTHrP. J Clin Invest. 2005; 115:1734–1742.
Article23. Shimoyama A, Wada M, Ikeda F, et al. Ihh/Gli2 signaling promotes osteoblast differentiation by regulating Runx2 expression and function. Mol Biol Cell. 2007; 18:2411–2418.
Article24. Yoshida CA, Furuichi T, Fujita T, et al. Core-binding factor beta interacts with Runx2 and is required for skeletal development. Nat Genet. 2002; 32:633–638.
Article25. Hata K, Nishimura R, Ueda M, et al. A CCAAT/enhancer binding protein beta isoform, liver-enriched inhibitory protein, regulates commitment of osteoblasts and adipocytes. Mol Cell Biol. 2005; 25:1971–1979.
Article26. Tominaga H, Maeda S, Hayashi M, et al. CCAAT/enhancer-binding protein beta promotes osteoblast differentiation by enhancing Runx2 activity with ATF4. Mol Biol Cell. 2008; 19:5373–5386.
Article27. Hirata M, Kugimiya F, Fukai A, et al. C/EBPbeta and RUNX2 cooperate to degrade cartilage with MMP-13 as the target and HIF-2alpha as the inducer in chondrocytes. Hum Mol Genet. 2012; 21:1111–1123.
Article28. Wang W, Lian N, Li L, et al. Atf4 regulates chondrocyte proliferation and differentiation during endochondral ossification by activating Ihh transcription. Development. 2009; 136:4143–4153.
Article29. Arnold MA, Kim Y, Czubryt MP, et al. MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev Cell. 2007; 12:377–389.
Article30. Kawane T, Komori H, Liu W, et al. Dlx5 and mef2 regulate a novel runx2 enhancer for osteoblast-specific expression. J Bone Miner Res. 2014; 29:1960–1969.
Article31. Saito T, Fukai A, Mabuchi A, et al. Transcriptional regulation of endochondral ossification by HIF-2alpha during skeletal growth and osteoarthritis development. Nat Med. 2010; 16:678–686.
Article32. Yang S, Kim J, Ryu JH, et al. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010; 16:687–693.
Article33. Nishimura R, Wakabayashi M, Hata K, et al. Osterix regulates calcification and degradation of chondrogenic matrices through matrix metalloproteinase 13 (MMP13) expression in association with transcription factor Runx2 during endochondral ossification. J Biol Chem. 2012; 287:33179–33190.
Article34. Kamekura S, Kawasaki Y, Hoshi K, et al. Contribution of runt-related transcription factor 2 to the pathogenesis of osteoarthritis in mice after induction of knee joint instability. Arthritis Rheum. 2006; 54:2462–2470.
Article35. Nakajima M, Shi D, Dai J, et al. Replication studies in various ethnic populations do not support the association of the HIF-2alpha SNP rs17039192 with knee osteoarthritis. Nat Med. 2011; 17:26–27. author reply 27-9.
Article36. Araldi E, Khatri R, Giaccia AJ, et al. Lack of HIF-2alpha in limb bud mesenchyme causes a modest and transient delay of endochondral bone development. Nat Med. 2011; 17:25–26. author reply 27-9.
Article37. Tsushima H, Okazaki K, Hayashida M, et al. CCAAT/enhancer binding protein beta regulates expression of matrix metalloproteinase-3 in arthritis. Ann Rheum Dis. 2012; 71:99–107.
Article38. Hayashida M, Okazaki K, Fukushi J, et al. CCAAT/enhancer binding protein beta mediates expression of matrix metalloproteinase 13 in human articular chondrocytes in inflammatory arthritis. Arthritis Rheum. 2009; 60:708–716.
Article39. Taniguchi Y, Kawata M, Chang SH, et al. Regulation of chondrocyte survival in mouse articular cartilage by p63. Arthritis Rheumatol. 2017; 69:598–609.
Article40. Hosaka Y, Saito T, Sugita S, et al. Notch signaling in chondrocytes modulates endochondral ossification and osteoarthritis development. Proc Natl Acad Sci U S A. 2013; 110:1875–1880.
Article41. Sugita S, Hosaka Y, Okada K, et al. Transcription factor Hes1 modulates osteoarthritis development in cooperation with calcium/calmodulin-dependent protein kinase 2. Proc Natl Acad Sci U S A. 2015; 112:3080–3085.
Article42. Hiramatsu K, Sasagawa S, Outani H, et al. Generation of hyaline cartilaginous tissue from mouse adult dermal fibroblast culture by defined factors. J Clin Invest. 2011; 121:640–657.
Article43. Yamashita A, Morioka M, Yahara Y, et al. Generation of scaffoldless hyaline cartilaginous tissue from human iPSCs. Stem Cell Reports. 2015; 4:404–418.
Article44. Rhee DK, Marcelino J, Baker M, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005; 115:622–631.
Article45. Asahara H. Current status and strategy of microRNA research for cartilage development and osteoarthritis pathogenesis. J Bone Metab. 2016; 23:121–127.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Status and Strategy of microRNA Research for Cartilage Development and Osteoarthritis Pathogenesis
- SoxD Transcription Factors: Multifaceted Players of Neural Development
- Regulation of NFATc1 in Osteoclast Differentiation
- Transcriptional Regulation of Fibroblast Growth Factor 21 Expression
- Neurogenesis and Regulation of Olfactory Epithelium