Yonsei Med J.
2013 Nov;54(6):1407-1415. 10.3349/ymj.2013.54.6.1407.
Human Leptin Protein Induces Proliferation of A549 Cells via Inhibition of PKR-Like ER Kinase and Activating Transcription Factor-6 Mediated Apoptosis
- Affiliations
-
- 1Department of Thoracic Surgery, General Hospital of Zaozhuang Mining Group, Shandong, Zaozhuang, China. laiqunzz@yeah.net
- KMID: 1798137
- DOI: http://doi.org/10.3349/ymj.2013.54.6.1407
Abstract
- PURPOSE
To investigate the anti-apoptotic mechanism of leptin in non-small cell lung cancer.
MATERIALS AND METHODS
The influences of leptin on apoptosis were investigated, analyzing the mechanism that triggers growth of A549 cells. The effects of leptin on cell proliferation were examined by XTT analysis. Leptin, C/EBP homologous protein (CHOP), phosphorylated-PKR-like ER kinase (p-Perk), inositol requiring proteins-1, spliced X-box transcription factor-1 (XBP1), cleaved activating transcription factor-6 (ATF6), eukaryotic translation initiation factor-2alpha, caspase-12 and CHOP protein were detected in four groups by western blot, and endoplasmic reticulum (ER) stress related mRNA were detected by reverse transcription PCR.
RESULTS
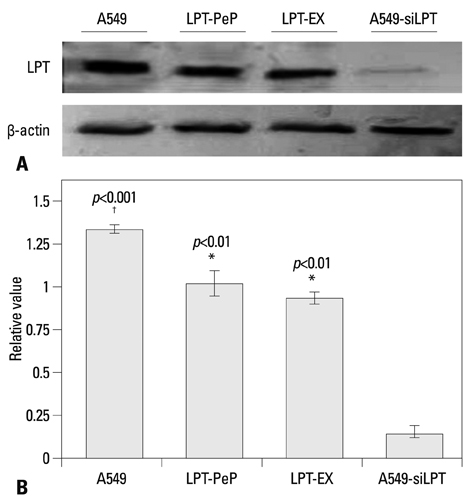
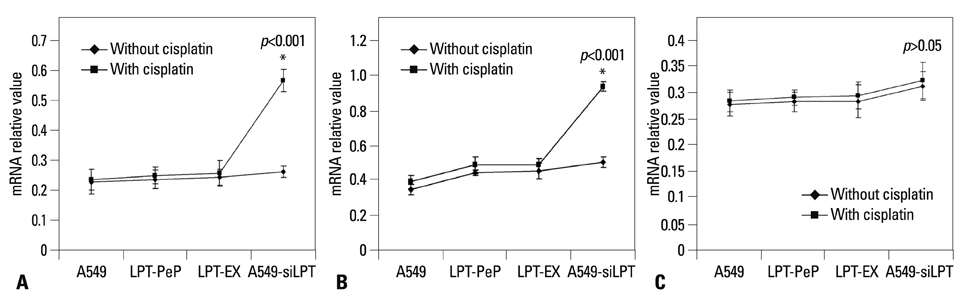
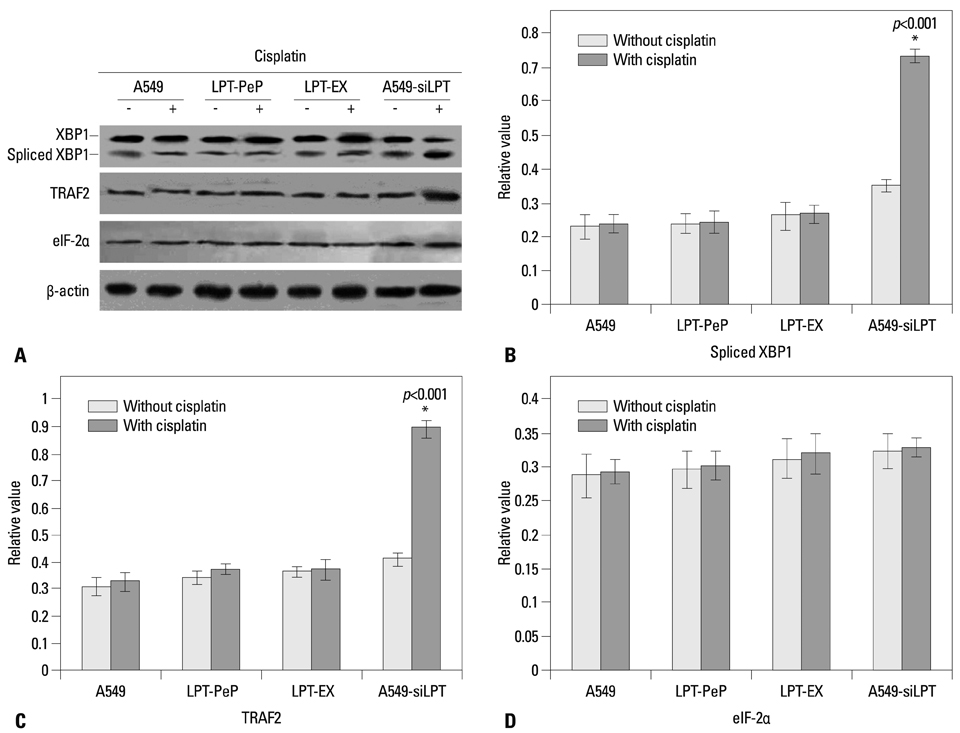
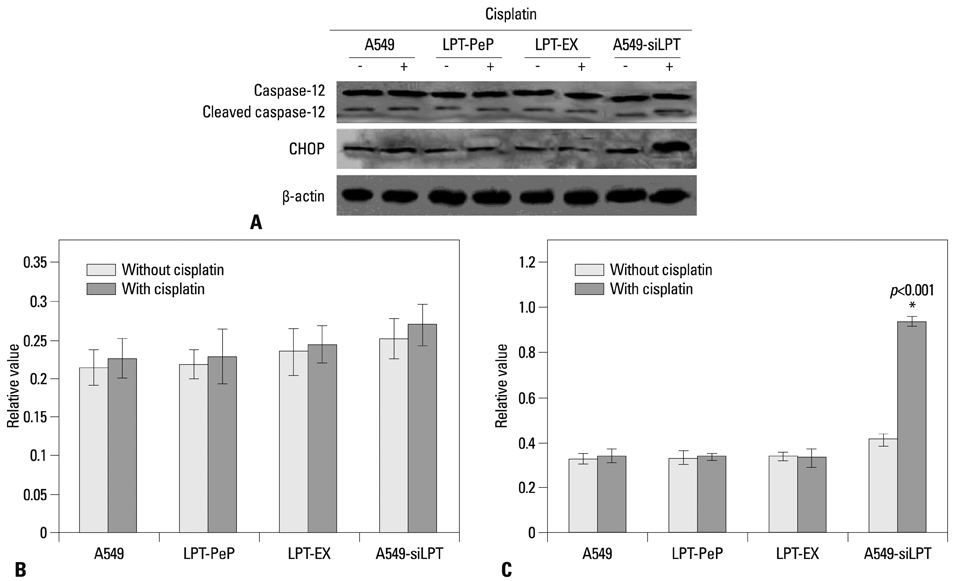
The expression of leptin in A549 and leptin transfected cells inhibited cisplatin activated ER stress-associated mRNA transcription and protein activation. Two ER stress unfolded protein response pathways, PERK and ATF6, were involved, and XBP1 and tumor necrosis factor receptor-associated factor 2 (TRAF2) were increased significantly when treated with cisplatin in A549-siRNA against leptin cells. Furthermore, CHOP expression was inhibited upon leptin expression in A549, LPT-PeP and LPT-EX cells.
CONCLUSION
Leptin serves as an important factor that promotes the growth of A549 cells through blocking ER stress-mediated pathways. This blocking is triggered by p-Perk and ATF6 via inhibition of CHOP expression.
MeSH Terms
Figure
Reference
-
1. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008; 83:584–594.
Article2. Petersen I, Petersen S. Towards a genetic-based classification of human lung cancer. Anal Cell Pathol. 2001; 22:111–121.
Article3. Al Husaini H, Wheatley-Price P, Clemons M, Shepherd FA. Prevention and management of bone metastases in lung cancer: a review. J Thorac Oncol. 2009; 4:251–259.
Article4. Yung HW, Korolchuk S, Tolkovsky AM, Charnock-Jones DS, Burton GJ. Endoplasmic reticulum stress exacerbates ischemia-reperfusion-induced apoptosis through attenuation of Akt protein synthesis in human choriocarcinoma cells. FASEB J. 2007; 21:872–884.
Article5. Moenner M, Pluquet O, Bouchecareilh M, Chevet E. Integrated endoplasmic reticulum stress responses in cancer. Cancer Res. 2007; 67:10631–10634.
Article6. Feldman DE, Chauhan V, Koong AC. The unfolded protein response: a novel component of the hypoxic stress response in tumors. Mol Cancer Res. 2005; 3:597–605.
Article7. Joung KH, Cho SC. Stress responses of neonates related to maternal characteristics. Yonsei Med J. 2011; 52:98–103.
Article8. Momoi T, Fujita E, Senoo H, Momoi M. Genetic factors and epigenetic factors for autism: endoplasmic reticulum stress and impaired synaptic function. Cell Biol Int. 2009; 34:13–19.9. Hung JY, Hsu YL, Ni WC, Tsai YM, Yang CJ, Kuo PL, et al. Oxidative and endoplasmic reticulum stress signaling are involved in dehydrocostuslactone-mediated apoptosis in human non-small cell lung cancer cells. Lung Cancer. 2010; 68:355–365.
Article10. Terzidis A, Sergentanis TN, Antonopoulos G, Syrigos C, Efremidis A, Polyzos A, et al. Elevated serum leptin levels: a risk factor for non-small-cell lung cancer? Oncology. 2009; 76:19–25.
Article11. Caldefie-Chézet F, Damez M, de Latour M, Konska G, Mishellani F, Fusillier C, et al. Leptin: a proliferative factor for breast cancer? Study on human ductal carcinoma. Biochem Biophys Res Commun. 2005; 334:737–741.12. Geng Y, Wang J, Wang R, Wang K, Xu Y, Song G, et al. Leptin and HER-2 are associated with gastric cancer progression and prognosis of patients. Biomed Pharmacother. 2012; 66:419–424.
Article13. Wang X, Dong CF, Shi Q, Shi S, Wang GR, Lei YJ, et al. Cytosolic prion protein induces apoptosis in human neuronal cell SH-SY5Y via mitochondrial disruption pathway. BMB Rep. 2009; 42:444–449.
Article14. Xuan B, Qian Z, Torigoi E, Yu D. Human cytomegalovirus protein pUL38 induces ATF4 expression, inhibits persistent JNK phosphorylation, and suppresses endoplasmic reticulum stress-induced cell death. J Virol. 2009; 83:3463–3474.
Article15. Wang Q, He Z, Zhang J, Wang Y, Wang T, Tong S, et al. Overexpression of endoplasmic reticulum molecular chaperone GRP94 and GRP78 in human lung cancer tissues and its significance. Cancer Detect Prev. 2005; 29:544–551.
Article16. Chérasse Y, Maurin AC, Chaveroux C, Jousse C, Carraro V, Parry L, et al. The p300/CBP-associated factor (PCAF) is a cofactor of ATF4 for amino acid-regulated transcription of CHOP. Nucleic Acids Res. 2007; 35:5954–5965.
Article17. Wang X, Shi Q, Xu K, Gao C, Chen C, Li XL, et al. Familial CJD associated PrP mutants within transmembrane region induced Ctm-PrP retention in ER and triggered apoptosis by ER stress in SH-SY5Y cells. PLoS One. 2011; 6:e14602.
Article18. Moon DO, Park SY, Choi YH, Ahn JS, Kim GY. Guggulsterone sensitizes hepatoma cells to TRAIL-induced apoptosis through the induction of CHOP-dependent DR5: involvement of ROS-dependent ER-stress. Biochem Pharmacol. 2011; 82:1641–1650.
Article19. Tagawa Y, Hiramatsu N, Kasai A, Hayakawa K, Okamura M, Yao J, et al. Induction of apoptosis by cigarette smoke via ROS-dependent endoplasmic reticulum stress and CCAAT/enhancer-binding protein-homologous protein (CHOP). Free Radic Biol Med. 2008; 45:50–59.
Article20. Kim KM, Kim HC, Jeon KN, Kim HG, Kang JH, Hahm JR, et al. Rituximab-CHOP induced interstitial pneumonitis in patients with disseminated extranodal marginal zone B cell lymphoma. Yonsei Med J. 2008; 49:155–158.
Article21. Wang SC, Lu MC, Chen HL, Tseng HI, Ke YY, Wu YC, et al. Cytotoxicity of calotropin is through caspase activation and downregulation of anti-apoptotic proteins in K562 cells. Cell Biol Int. 2009; 33:1230–1236.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Kahweol from Coffee Induces Apoptosis by Upregulating Activating Transcription Factor 3 in Human Colorectal Cancer Cells
- Naringenin-Mediated ATF3 Expression Contributes to Apoptosis in Human Colon Cancer
- HIV-1 Infection Causes Intracellular Expression of p53, Which Induces PKR Expression, Followed by Inhibition of HIV-1 Tat Activity
- Celecoxib induces cell death on non-small cell lung cancer cells through endoplasmic reticulum stress
- A Novel Type of Non-coding RNA, nc886, Implicated in Tumor Sensing and Suppression