J Korean Surg Soc.
2011 Oct;81(4):235-241. 10.4174/jkss.2011.81.4.235.
Mycophenolic acid mediated mitochondrial membrane potential transition change lead to T lymphocyte apoptosis
- Affiliations
-
- 1Department of Surgery, Chonnam National University Medical School, Gwangju, Korea. sycpvts@chonnam.ac.kr
- 2Department of Interanl Medicine, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 1445743
- DOI: http://doi.org/10.4174/jkss.2011.81.4.235
Abstract
- PURPOSE
This study demonstrated that apoptosis induced by mycophenolic acid (MPA) is mediated by mitochondrial membrane potential transition (MPT) changes in Jurkat cells.
METHODS
Cell viability and MPT changes were measured by flow cytometry. Western blotting was performed to evaluate the expression of Bcl-2 family proteins, Bid, truncated Bid (tBid), cytochrome c, voltage dependent anion channel (VDAC), poly ADP-ribose polymerase (PARP), and protein kinase C-delta (PKC-delta). The catalytic activity of caspase-9 and -3 was also measured.
RESULTS
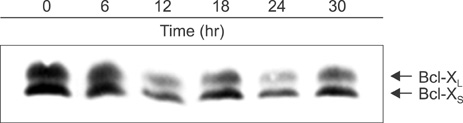
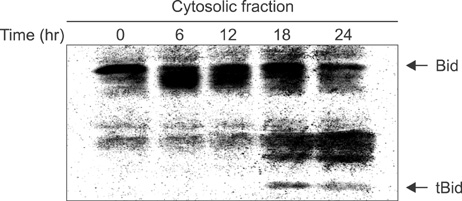
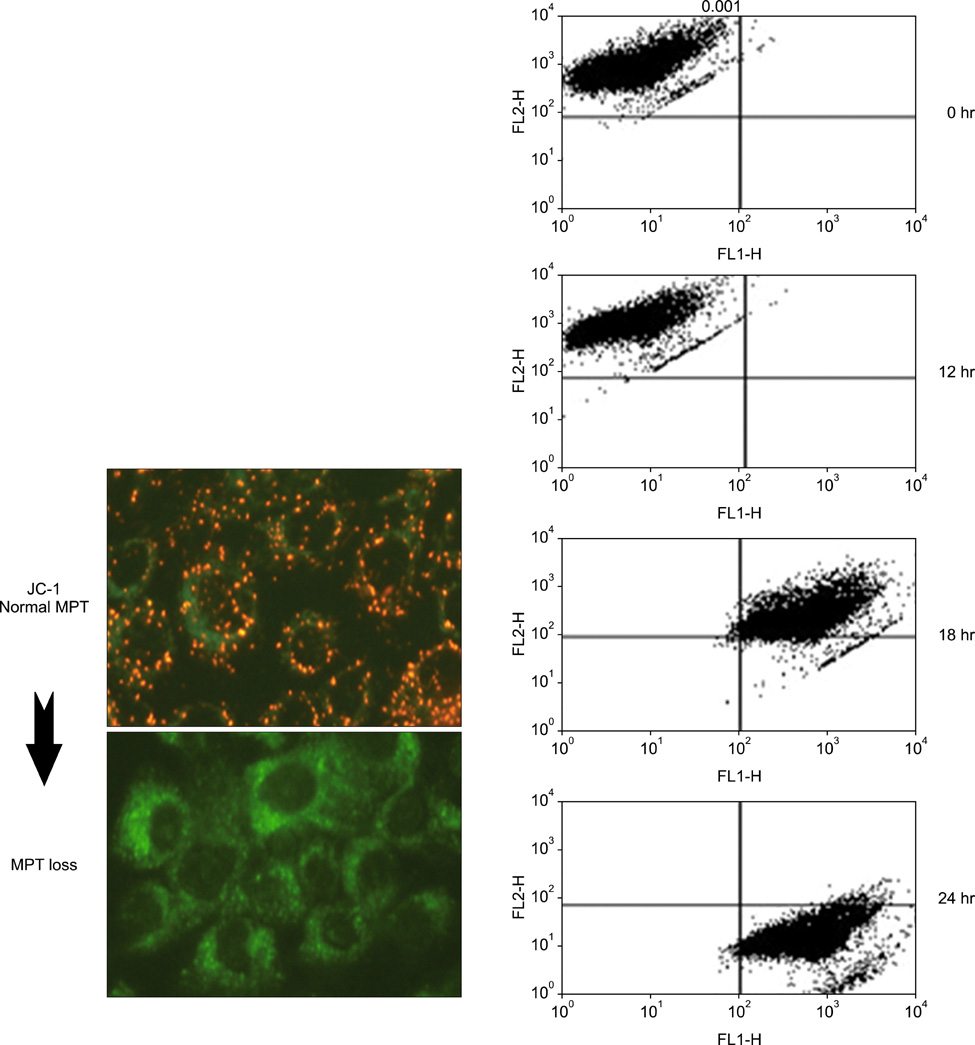
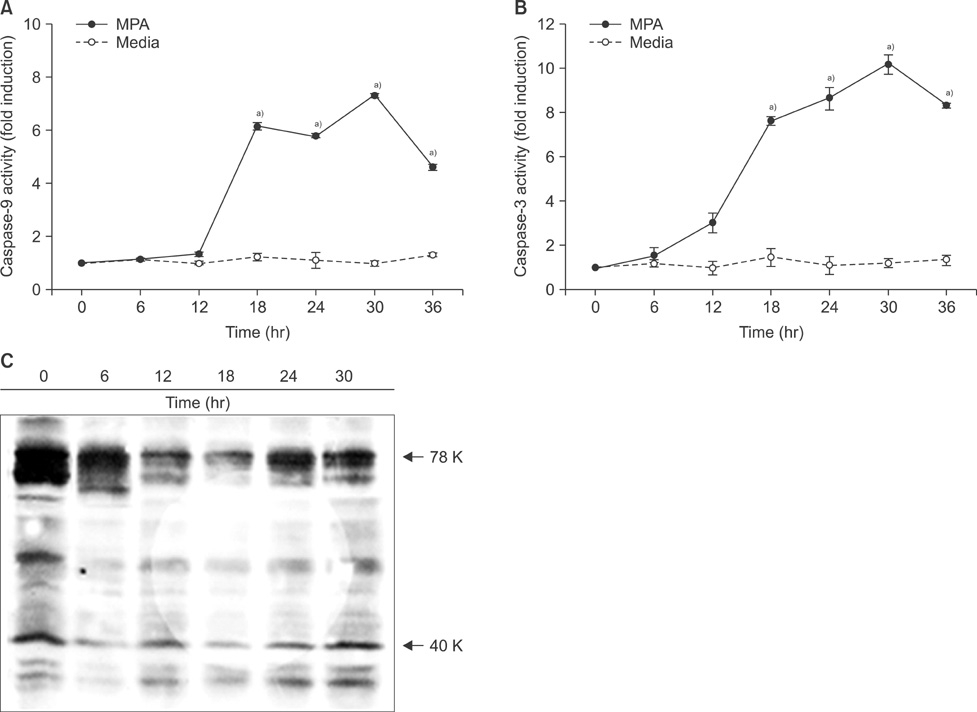
Cell viability was decreased in time- and dose-dependent manners. Bcl-2 protein expression was decreased, but Bax protein expression was identified. A decreased Bcl-XL /Bcl-XS ratio was also noted. The expression of tBid protein also increased in a time-dependent manner in Jurkat cells treated with MPA. While normal MPT appeared as orange fluorescence, abnormal MPT corresponded to green fluorescence. Green fluorescence increased as orange decreased in the MPA-treated cells. Significantly increased concentrations of MPA induced the release of cytosolic cytochrome c. MPA also augmented the catalytic activity of caspase-9 and caspase-3 in Jurkat cells. Our findings demonstrated that MPA-induced apoptosis is mediated by MPT changes accompanied by decreased Bcl-XL expression and the appearance of tBid protein. The release of cytosolic cytochrome c from mitochondria and increased catalytic activity of caspase-9 and caspase-3 were observed in MPA-treated Jurkat cells.
CONCLUSION
These results suggest that mitochondrial dysfunction caused by MPA induces human T lymphocyte apoptosis.
MeSH Terms
-
Adenosine Diphosphate Ribose
Apoptosis
bcl-2-Associated X Protein
BH3 Interacting Domain Death Agonist Protein
Blotting, Western
Caspase 3
Caspase 9
Cell Survival
Citrus sinensis
Cytochromes c
Cytosol
Flow Cytometry
Fluorescence
Humans
Jurkat Cells
Lymphocytes
Membrane Potential, Mitochondrial
Mitochondria
Mitochondrial Membranes
Mycophenolic Acid
Protein Kinase C-delta
Proteins
Adenosine Diphosphate Ribose
BH3 Interacting Domain Death Agonist Protein
Caspase 3
Caspase 9
Cytochromes c
Mycophenolic Acid
Protein Kinase C-delta
Proteins
bcl-2-Associated X Protein
Figure
Reference
-
1. Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999. 13:1899–1911.2. Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990. 348:334–336.3. Crompton M. Bax, Bid and the permeabilization of the mitochondrial outer membrane in apoptosis. Curr Opin Cell Biol. 2000. 12:414–419.4. Lee WA, Gu L, Miksztal AR, Chu N, Leung K, Nelson PH. Bioavailability improvement of mycophenolic acid through amino ester derivatization. Pharm Res. 1990. 7:161–166.5. Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S, Peter ME. Apoptosis signaling by death receptors. Eur J Biochem. 1998. 254:439–459.6. Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002. 2:647–656.7. Vander Heiden MG, Chandel NS, Williamson EK, Schumacker PT, Thompson CB. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997. 91:627–637.8. Annis MG, Soucie EL, Dlugosz PJ, Cruz-Aguado JA, Penn LZ, Leber B, et al. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J. 2005. 24:2096–2103.9. Huang DC, Strasser A. BH3-Only proteins-essential initiators of apoptotic cell death. Cell. 2000. 103:839–842.10. Lee YC, Kim JJ, Park HR, Lee S, Woo YM, Cho MH, et al. A study for apoptosis and its mechanism of allogeneic activated T lymphocytes induced by mouse liver immature dendritic cells. J Korean Surg Soc. 2004. 66:1–4.11. Li PF, Dietz R, von Harsdorf R. p53 regulates mitochondrial membrane potential through reactive oxygen species and induces cytochrome c-independent apoptosis blocked by Bcl-2. EMBO J. 1999. 18:6027–6036.12. Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000. 10:369–377.13. Gogvadze V, Orrenius S, Zhivotovsky B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim Biophys Acta. 2006. 1757:639–647.14. Hirata H, Takahashi A, Kobayashi S, Yonehara S, Sawai H, Okazaki T, et al. Caspases are activated in a branched protease cascade and control distinct downstream processes in Fas-induced apoptosis. J Exp Med. 1998. 187:587–600.15. Creagh EM, Martin SJ. Caspases: cellular demolition experts. Biochem Soc Trans. 2001. 29(Pt 6):696–702.16. Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999. 15:269–290.17. Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999. 397:441–446.18. Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998. 281:1309–1312.19. Ly JD, Grubb DR, Lawen A. The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis. 2003. 8:115–128.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Eugenol Induces a Reactive Oxygen Species-mediated Apoptosis in HL-60 Human Promyelocytic Leukemia Cells
- Genistein-Induced Apoptosis of p815 Mastocytoma Cell
- Effects of Heme Oxygenase-1 Expression in Mycophenolic Acid Induced Apoptosis of Jurkat Cell Lines
- Study on the Mitochondrial Dysfunction by p53 Regulation in Ceramide-induced Neuronal Cell Death
- CM1 Ligation Induces Apoptosis via Fas-FasL Interaction in Ramos Cells, but via Down-regulation of Bcl-2 and Subsequent Decrease of Mitochondrial Membrane Potential in Raji Cells