Lab Anim Res.
2012 Jun;28(2):123-129. 10.5625/lar.2012.28.2.123.
Anti-hyperlipidemic effect of soybean extract fermented by Bacillus subtilis MORI in db/db mice
- Affiliations
-
- 1Institute of Natural Medicine, Hallym University, Chuncheon, Korea. jgsuh@hallym.ac.kr
- 2Department of Medical Genetics, College of Medicine, Hallym University, Chuncheon, Korea.
- 3R&D Center for Life Science, Biotopia Co., Ltd., Chuncheon, Korea.
- 4Department of Life Science, University of Suwon, Hwaseong, Korea.
- KMID: 1436709
- DOI: http://doi.org/10.5625/lar.2012.28.2.123
Abstract
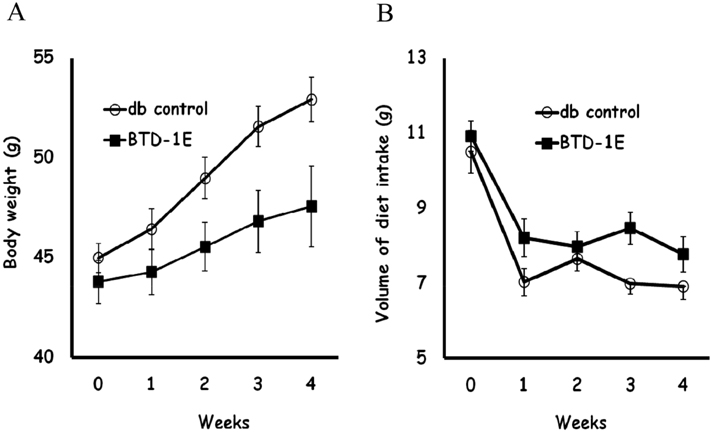
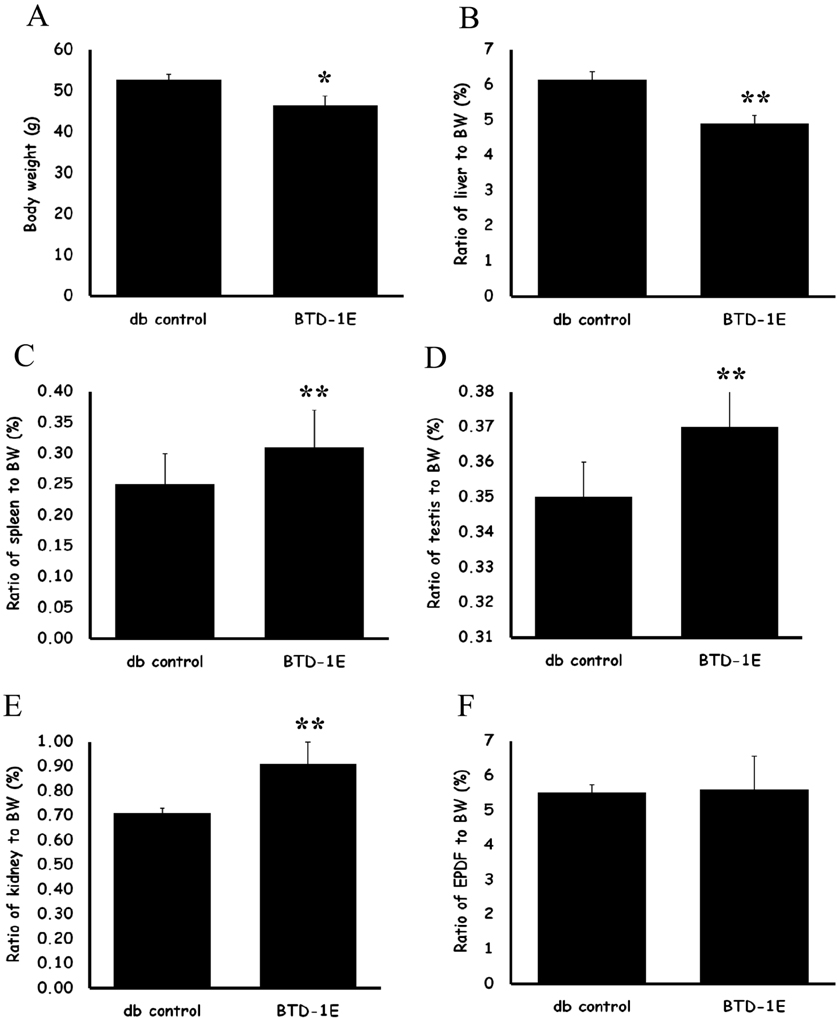
- The purpose of this study was to investigate the anti-hyperlipidemic effect of soy bean extract solution fermented by Bacillus subtilis MORI (BTD-1E) in obese db/db mice. Eight-week-old male db/db mice were administered 33.3 mg/kg BTD-1E solution orally once a day for four weeks. The BTD-1E group showed significantly lower body weight compared with the db control group (P<0.05). The BTD-1E group showed significantly lower serum total cholesterol and LDL cholesterol levels compared with the db control group, respectively (P<0.05, P<0.01). The BTD-1E group showed significantly decreased liver weight relative to final body weight compared with the db control group (P<0.01). After four weeks of BTD-1E administration, lipid droplets in the liver were apparently decreased in the BTD-1E group compared to the db control group. In summary, our results suggest that BTD-1E has an anti-hyperlipidemic effect in the obese mouse model.
Keyword
MeSH Terms
Figure
Reference
-
1. Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004. 291(23):2847–2850.2. Strader CD, MacIntyre DE, Candelore MR, Parker EM. Molecular approaches to the discovery of new treatments for obesity. Curr Opin Chem Biol. 1997. 1(2):204–209.3. Kim Y, Park YJ, Yang SO, Kim SH, Hyun SH, Cho S, Kim YS, Kwon DY, Cha YS, Chae S, Choi HK. Hypoxanthine levels in human urine serve as a screening indicator for the plasma total cholesterol and low-density lipoprotein modulation activities of fermented red pepper paste. Nutr Res. 2010. 30(7):455–461.4. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999. 282(16):1523–1529.5. Kopelman PG. Obesity as a medical problem. Nature. 2000. 404(6778):635–643.6. Lew EA. Mortality and weight: insured lives and the American Cancer Society studies. Ann Intern Med. 1985. 103:1024–1029.7. Kang NH, Lee WK, Yi BR, Park MA, Lee HR, Park SK, Hwang KA, Park HK, Choi KC. Modulation of lipid metabolism by mixtures of protamine and chitooligosaccharide through pancreatic lipase inhibitory activity in a rat model. Lab Anim Res. 2012. 28(1):31–38.8. Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet. 2007. 369(9555):71–77.9. Bays HE. Current and investigational antiobesity agents and obesity therapeutic treatment targets. Obes Res. 2004. 12(8):1197–1211.10. Hauptman JB, Jeunet FS, Hartmann D. Initial studies in humans with the novel gastrointestinal lipase inhibitor Ro 18-0647 (tetrahydrolipstatin). Am J Clin Nutr. 1992. 55:1 Suppl. 309S–313S.11. McNeely W, Goa KL. Sibutramine. A review of its contribution to the management of obesity. Drugs. 1998. 56(6):1093–1124.12. Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Néliat G, Caput D, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994. 350(2-3):240–244.13. Padwal R, Li SK, Lau DC. Long-term pharmacotherapy for obesity and overweight. Cochrane Database Syst Rev. 2003. 4:CD004094.14. Wooltorton E. Obesity drug sibutramine (Meridia): hypertension and cardiac arrhythmias. CMAJ. 2002. 166(10):1307–1308.15. Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet. 2007. 369(9555):71–77.16. Nakanishi H, Onose S, Kitahara E, Chumchuen S, Takasaki M, Konishi H, Kanekatsu R. Effect of environmental conditions on the α-glucosidase inhibitory activity of mulberry leaves. Biosci Biotechnol Biochem. 2011. 75(12):2293–2296.17. Tsuduki T, Nakamura Y, Honma T, Nakagawa K, Kimura T, Ikeda I, Miyazawa T. Intake of 1-deoxynojirimycin suppresses lipid accumulation through activation of the beta-oxidation system in rat liver. J Agric Food Chem. 2009. 57(22):11024–11029.18. Ezure Y, maruo S, Miyazaki K, kawamata M. Moranoline (1-Deoxynojirimycin) fermentation and its improvement. Agric Biol Chem. 1985. 49(4):1119–1125.19. Bischoff H. The mechanism of alpha-glucosidase inhibition in the management of diabetes. Clin Invest Med. 1995. 18(4):303–311.20. Inoue I, Shinoda Y, Nakano T, Sassa M, Goto S, Awata T, Komoda T, Katayama S. Acarbose ameliorates atherogenecity of low-density lipoprotein in patients with impaired glucose tolerance. Metabolism. 2006. 55(7):946–952.21. Cheik NC, Rossi EA, Guerra RL, Tenorio NM, Oller do Nascimento CM, Viana FP, Manzoni MS, Carlos IZ, Leao da Silva P, Vendramini RC, Damaso AR. Effects of a ferment soy product on the adipocyte area reduction and dyslipidemia control in hypercholesterolemic adult male rats. Lipids Health Dis. 2008. 7:50.22. Nojima H, Kimura I, Chen FJ, Sugihara Y, Haruno M, Kato A, Asano N. Antihyperglycemic effects of N-containing sugars from Xanthocercis zambesiaca, Morus bombycis, Aglaonema treubii, and Castanospermum australe in streptozotocin-diabetic mice. J Nat Prod. 1998. 61(3):397–400.23. Kimura T, Nakagawa K, Kubota H, Kojima Y, Goto Y, Yamagishi K, Oita S, Oikawa S, Miyazawa T. Food-grade mulberry powder enriched with 1-deoxynojirimycin suppresses the elevation of postprandial blood glucose in humans. J Agric Food Chem. 2007. 55(14):5869–5874.24. Kim HS, Lee JY, Hwang KY, Cho YS, Park YS, Kang KD, Seong SI. Isolation and identification of a Bacillus sp. producing alpha-glucosidase inhibitor 1-deoxynojirimycin. Korean J Microbiol Biotechnol. 2011. 39(1):49–55.25. Chae JY, Lee JY, Hoang IS, Whangbo D, Choi PW, Lee WC, Kim JW, Kim SY, Choi SW, Rhee SJ. Analysis of functional components of leaves of different mulberry cultivars. J Korean Soc Food Sci Nutr. 2003. 32(1):15–21.26. Hwang KY, Kim YH, Cho YS, Park YS, Lee JY, Kang KD, Kim K, Joo DK, Ahn DK, Seong SI. Hypoglycemic effect of fermented soybean culture mixed with mulberry leaves on neonatal streptozotocin-induced diabetic rats. J Korean Soc Food Sci Nutr. 2008. 37(4):452–458.27. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995. 854:1–452.28. DeLany JP, Blohm F, Truett AA, Scimeca JA, West DB. Conjugated linoleic acid rapidly reduces body fat content in mice without affecting energy intake. Am J Physiol. 1999. 276(4):R1172–R1179.29. Flegal KM. Epidemiologic aspects of overweight and obesity in the United States. Physiol Behav. 2005. 86(5):599–602.30. Pyo YH, Seong KS. Hypolipidemic effects of Monascus-fermented soybean extracts in rats fed a high-fat and -cholesterol diet. J Agric Food Chem. 2009. 57(18):8617–8622.31. Song T, Lee SO, Murphy PA, Hendrich S. Soy protein with or without isoflavones, soy germ and soy germ extract, and daidzein lessen plasma cholesterol levels in golden Syrian hamsters. Exp Biol Med (Maywood). 2003. 228(9):1063–1068.32. Yousef MI, Kamel KI, Esmail AM, Baghdadi HH. Antioxidant activities and lipid lowering effects of isoflavone in male rabbits. Food Chem Toxicol. 2004. 42(9):1497–1503.33. Azain MJ, Hausman DB, Kasser TR, Martin RJ. Effect of somatotropin and feed restriction on body composition and adipose metabolism in obese Zucker rats. Am J Physiol. 1995. 269(1):E137–E144.34. Ikeda H, Shino A, Matsuo T, Iwatsuka H, Suzuoki Z. A new genetically obese-hyperglycemic rat (Wistar fatty). Diabetes. 1981. 30(12):1045–1050.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fermentation of purple Jerusalem artichoke extract to improve the α-glucosidase inhibitory effect in vitro and ameliorate blood glucose in db/db mice
- Evaluating the Allergenicity of Soybean by the Fermentation
- Sargassum coreanum extract alleviates hyperglycemia and improves insulin resistance in db/db diabetic mice
- Daraesoon (shoot of hardy kiwi) mitigates hyperglycemia in db/db mice by alleviating insulin resistance and inflammation
- Hematopoietic effect of deer antler extract fermented by Bacillus subtilis on murine marrow cells