Nutr Res Pract.
2016 Jun;10(3):282-287. 10.4162/nrp.2016.10.3.282.
Fermentation of purple Jerusalem artichoke extract to improve the α-glucosidase inhibitory effect in vitro and ameliorate blood glucose in db/db mice
- Affiliations
-
- 1Department of Food Science and Nutrition, Hallym University, 1 Hallymdeahak-gil, Chuncheon, 24252 Korea. limss@hallym.ac.kr
- 2Institute of Natural Medicine, Hallym University, Chuncheon, 24252 Korea.
- 3Natural Resources Commercialization, Chuncheon Bioindustry Foundation, Chuncheon, 24252 Korea.
- 4Frontbio Co. Ltd., Chuncheon, 24252 Korea.
- KMID: 2342130
- DOI: http://doi.org/10.4162/nrp.2016.10.3.282
Abstract
- BACKGROUND/OBJECTIVES
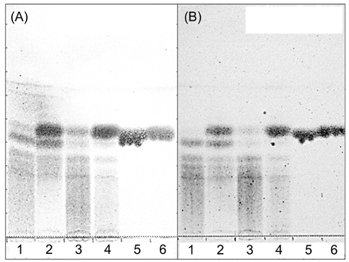
Jerusalem artichoke has inhibitory activity against α-glucosidase and decreases fasting serum glucose levels, which may be related to its fructan content. The biological activity of fructan can be influenced by the degree of polymerization. Thus, in this study, the inhibitory effects of original and fermented purple Jerusalem artichoke (PJA) on α-glucosidase were compared in vitro. Additionally, the anti-diabetes effect of Lactobacillus plantarum-fermented PJA (LJA) was studied in a non-insulin-dependent diabetes mellitus animal model (C57BIKsJ db/db).
MATERIALS/METHODS
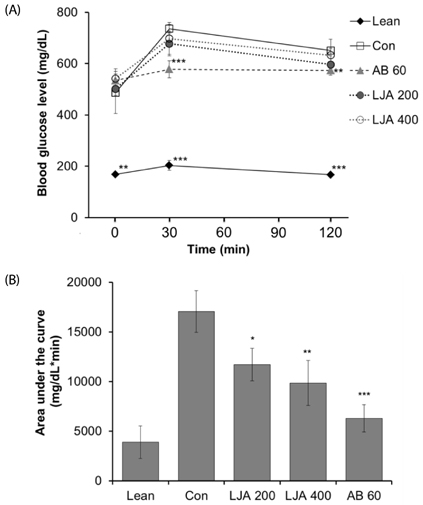
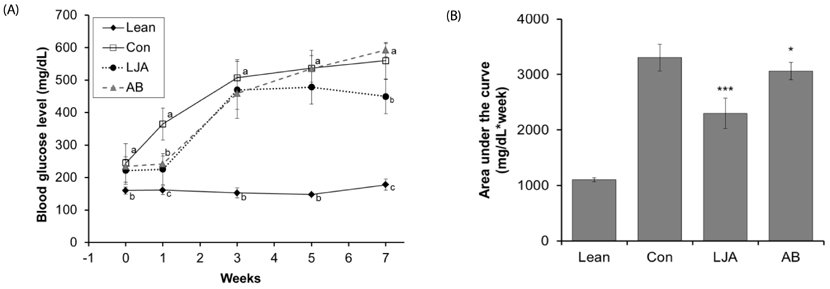
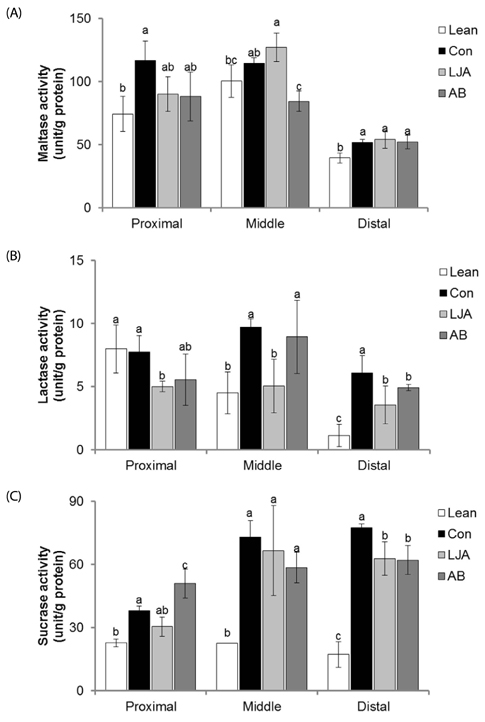
The water extract of PJA was fermented by L. plantarum, and two strains of Bacillus subtilis to compare their anti-α-glucosidase activities in vitro by α-glucosidase assays. The anti-diabetes effect of LJA was studied in a non-insulin-dependent diabetes mellitus animal model (C57BIKsJ db/db) for seven weeks. During the experiment, food intake, body weight, and fasting blood glucose were measured every week. At the end of the treatment period, several diabetic parameters and the intestinal α-glucosidase activity were measured.
RESULTS
The LJA showed the highest α-glucosidase inhibitory activity in vitro. In the in vivo study, it resulted in a significantly lower blood glucose concentration than the control. Serum insulin and HDL cholesterol levels were significantly higher and the concentrations of triglycerides, non-esterified fatty acids, and total cholesterol were significant lower in mice treated with LJA after seven weeks. In addition, the intestinal α-glucosidase activity was partially inhibited.
CONCLUSIONS
These results suggested that LJA regulates blood glucose and has potential use as a dietary supplement.
MeSH Terms
-
Animals
Bacillus subtilis
Blood Glucose*
Body Weight
Cholesterol
Cholesterol, HDL
Diabetes Mellitus
Diabetes Mellitus, Type 2
Dietary Supplements
Eating
Fasting
Fatty Acids
Fermentation*
Helianthus*
In Vitro Techniques*
Insulin
Lactobacillus
Lactobacillus plantarum
Mice*
Models, Animal
Polymerization
Polymers
Triglycerides
Water
Blood Glucose
Cholesterol
Cholesterol, HDL
Fatty Acids
Insulin
Polymers
Triglycerides
Water
Figure
Reference
-
1. Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus--present and future perspectives. Nat Rev Endocrinol. 2011; 8:228–236.
Article2. Fuller JH, Shipley MJ, Rose G, Jarrett RJ, Keen H. Coronary-heart-disease risk and impaired glucose tolerance. The Whitehall study. Lancet. 1980; 1:1373–1376.
Article3. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010; 33:Suppl 1. S62–S69.4. Krasikov VV, Karelov DV, Firsov LM. α-Glucosidases. Biochemistry (Mosc). 2001; 66:267–281.5. Scheen AJ. Is there a role for α-glucosidase inhibitors in the prevention of type 2 diabetes mellitus? Drugs. 2003; 63:933–951.
Article6. Holman RR, Turner RC. Oral agents and insulin in the treatment of NIDDM. In : Pickup JC, Williams G, editors. Textbook of Diabetes. Oxford: Blackwell;1991. p. 467–469.7. Kim SH, Hyun SH, Choung SY. Anti-diabetic effect of cinnamon extract on blood glucose in db/db mice. J Ethnopharmacol. 2006; 104:119–123.
Article8. Rojo LE, Ribnicky D, Logendra S, Poulev A, Rojas-Silva P, Kuhn P, Dorn R, Grace MH, Lila MA, Raskin I. In vitro and in vivo anti-diabetic effects of anthocyanins from Maqui Berry (Aristotelia chilensis). Food Chem. 2012; 131:387–396.
Article9. Chernenko TV, Glushenkova AI, Rakhimov DA. Lipids of Helianthus tuberosus tubers. Chem Nat Compd. 2008; 44:1–2.
Article10. Kim HS, Han GD. Hypoglycemic and hepatoprotective effects of Jerusalem artichoke extracts on streptozotocin-induced diabetic rats. Food Sci Biotechnol. 2013; 22:1121–1124.
Article11. Rumessen JJ, Bodé S, Hamberg O, Gudmand-Høyer E. Fructans of Jerusalem artichokes: intestinal transport, absorption, fermentation, and influence on blood glucose, insulin, and C-peptide responses in healthy subjects. Am J Clin Nutr. 1990; 52:675–681.
Article12. Park BS. Effect of oral administration of Jerusalem artichoke inulin on reducing blood lipid and glucose in STZ-induced diabetic rats. J Anim Vet Adv. 2011; 10:2501–2507.
Article13. Kaur N, Gupta AK. Applications of inulin and oligofructose in health and nutrition. J Biosci. 2002; 27:703–714.
Article14. Mohamed Sham Shihabudeen H, Hansi Priscilla D, Thirumurugan K. Cinnamon extract inhibits α-glucosidase activity and dampens postprandial glucose excursion in diabetic rats. Nutr Metab (Lond). 2011; 8:46.
Article15. Barham D, Trinder P. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst. 1972; 97:142–145.
Article16. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193:265–275.
Article17. Jung HW, Jung JK, Ramalingam M, Yoon CH, Bae HS, Park YK. Anti-diabetic effect of Wen-pi-tang-Hab-Wu-ling-san extract in streptozotocin-induced diabetic rats. Indian J Pharmacol. 2012; 44:97–102.
Article18. Baldini M, Danuso F, Turi M, Vannozzi GP. Evaluation of new clones of Jerusalem artichoke (Helianthus tuberosus L.) for inulin and sugar yield from stalks and tubers. Ind Crops Prod. 2004; 19:25–40.
Article19. Rakhimov DA, Arifkhodzhaev AO, Mezhlumyan LG, Yuldashev OM, Rozikova UA, Aikhodzhaeva N, Vakil MM. Carbohydrates and proteins from Helianthus tuberosus. Chem Nat Compd. 2003; 39:312–313.
Article20. Suseelan KN, Mitra R, Pandey R, Sainis KB, Krishna TG. Purification and characterization of a lectin from wild sunflower (Helianthus tuberosus L.) tubers. Arch Biochem Biophys. 2002; 407:241–247.
Article21. Boillot J, Alamowitch C, Berger AM, Luo J, Bruzzo F, Bornet FR, Slama G. Effects of dietary propionate on hepatic glucose production, whole-body glucose utilization, carbohydrate and lipid metabolism in normal rats. Br J Nutr. 1995; 73:241–251.
Article22. Luo J, Rizkalla SW, Alamowitch C, Boussairi A, Blayo A, Barry JL, Laffitte A, Guyon F, Bornet FR, Slama G. Chronic consumption of short-chain fructooligosaccharides by healthy subjects decreased basal hepatic glucose production but had no effect on insulinstimulated glucose metabolism. Am J Clin Nutr. 1996; 63:939–945.
Article23. Yang HJ, Kwon DY, Kim MJ, Kang S, Kim DS, Park S. Jerusalem artichoke and chungkookjang additively improve insulin secretion and sensitivity in diabetic rats. Nutr Metab (Lond). 2012; 9:112.
Article24. Delzenne NM, Kok N. Effect of non-digestible fermentable carbohydrates on hepatic fatty acid metabolism. Biochem Soc Trans. 1998; 26:228–230.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Autumn olive (Elaeagnus umbellata Thunb.) berry reduces fasting and postprandial glucose levels in mice
- Sargassum coreanum extract alleviates hyperglycemia and improves insulin resistance in db/db diabetic mice
- Jerusalem Artichoke and Inulin
- Lotus leaf alleviates hyperglycemia and dyslipidemia in animal model of diabetes mellitus
- Effects of Jerusalem Artichoke Extract and Inulin on Blood Glucose Levels and Insulin Secretion in Streptozotocin Induced Diabetic Mice