Nutr Res Pract.
2019 Feb;13(1):11-16. 10.4162/nrp.2019.13.1.11.
Autumn olive (Elaeagnus umbellata Thunb.) berry reduces fasting and postprandial glucose levels in mice
- Affiliations
-
- 1Department of Smart Food and Drug, School of Bio Nano Information Technology, Inje University, 197 Inje-ro, Gimhae, Gyeongnam 50834, Korea. fdsnkiji@inje.ac.kr
- KMID: 2434071
- DOI: http://doi.org/10.4162/nrp.2019.13.1.11
Abstract
- BACKGROUND/OBJECTIVES
Fasting and postprandial hyperglycemia should be controlled to avoid complications of diabetes mellitus. This study investigated the effects of autumn olive (Elaeagnus umbellata Thunb.) berry (AOB) on fasting and postprandial hyperglycemia in mice.
MATERIALS/METHODS
In vitro α-glucosidase inhibitory effect of AOB was determined. Maltose solution (2 g/kg) with and without AOB extract at 500 mg/kg or acarbose at 50 mg/kg was orally administered to normal mice after overnight fasting and glucose levels were measured. To study the effects of chronic consumption of AOB, db/db mice received the basal diet or a diet containing AOB extract at 0.4% or 0.8%, or acarbose at 0.04% for 7 weeks. Blood glycated hemoglobin and serum glucose and insulin levels were measured. Expression of adiponectin protein in epididymal white adipose tissue was determined by Western blotting.
RESULTS
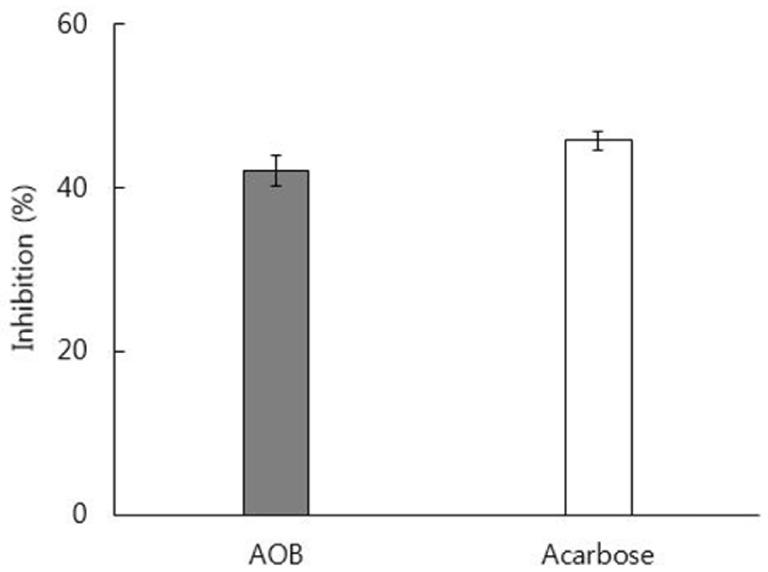
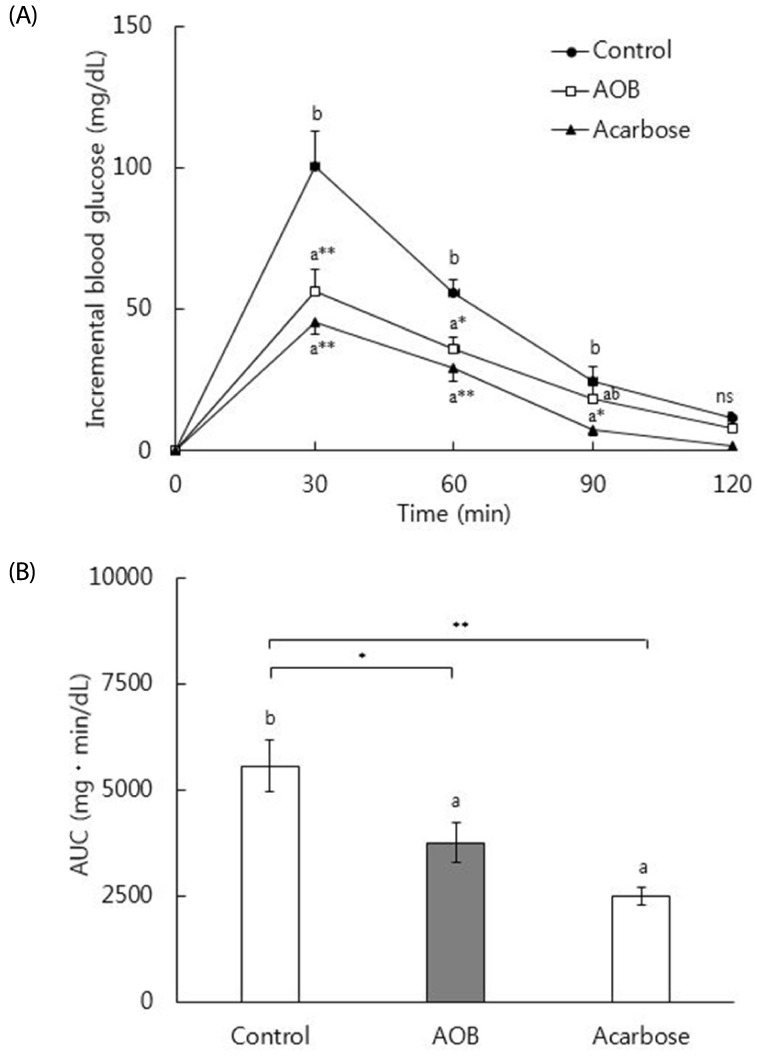
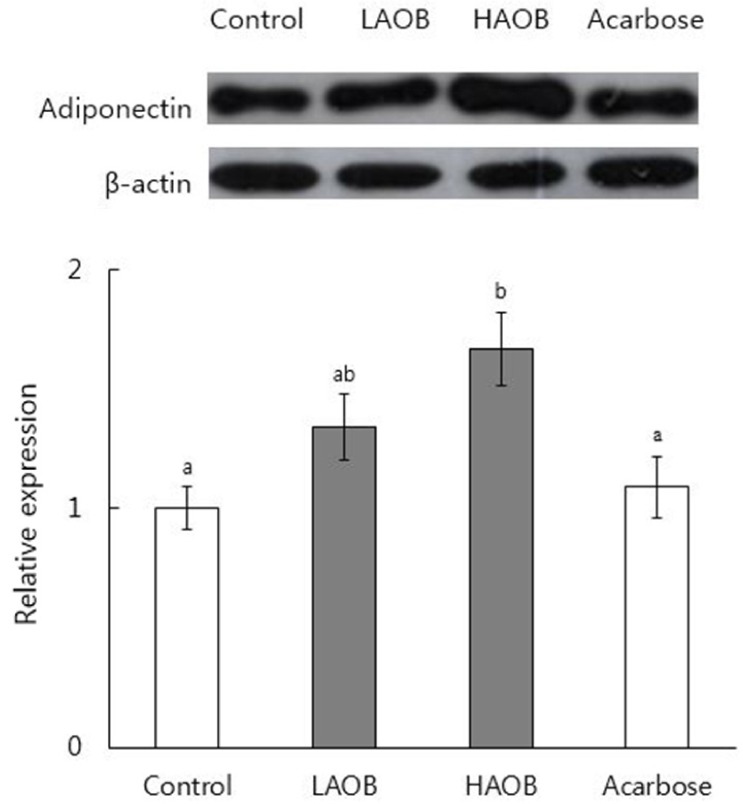
In vitro inhibitory effect of AOB extract on α-glucosidase was 92% as strong as that of acarbose. The AOB extract (500 mg/kg) or acarbose (50 mg/kg) significantly suppressed the postprandial rise of blood glucose after maltose challenge and the area under the glycemic response curve in normal mice. The AOB extract at 0.4% or 0.8% of diet or acarbose at 0.04% of diet significantly lowered levels of serum glucose and blood glycated hemoglobin and homeostasis model assessment for insulin resistance values in db/db mice. The expression of adiponectin protein in adipose tissue was significantly elevated by the consumption of AOB at 0.8% of diet.
CONCLUSIONS
Autumn olive (E. umbellata Thunb.) berry may reduce postprandial hyperglycemia by inhibiting α-glucosidase in normal mice. Chronic consumption of AOB may alleviate fasting hyperglycemia in db/db mice partly by inhibiting α-glucosidase and upregulating adiponectin expression.
MeSH Terms
-
Acarbose
Adiponectin
Adipose Tissue
Adipose Tissue, White
Animals
Blood Glucose
Blotting, Western
Diabetes Complications
Diabetes Mellitus
Diet
Fasting*
Fruit*
Glucose*
Hemoglobin A, Glycosylated
Homeostasis
Hyperglycemia
In Vitro Techniques
Insulin
Insulin Resistance
Maltose
Mice*
Olea*
Acarbose
Adiponectin
Blood Glucose
Glucose
Insulin
Maltose
Figure
Reference
-
1. Zatalia SR, Sanusi H. The role of antioxidants in the pathophysiology, complications, and management of diabetes mellitus. Acta Med Indones. 2013; 45:141–147. PMID: 23770795.2. Cheng D. Prevalence, predisposition and prevention of type II diabetes. Nutr Metab (Lond). 2005; 2:29. PMID: 16232315.
Article3. King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998; 21:1414–1431. PMID: 9727886.
Article4. Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993; 16:434–444. PMID: 8432214.
Article5. Manson JE, Colditz GA, Stampfer MJ, Willett WC, Krolewski AS, Rosner B, Arky RA, Speizer FE, Hennekens CH. A prospective study of maturity-onset diabetes mellitus and risk of coronary heart disease and stroke in women. Arch Intern Med. 1991; 151:1141–1147. PMID: 2043016.
Article6. Bonora E, Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in type II diabetes: the epidemiological evidence. Diabetologia. 2001; 44:2107–2114. PMID: 11793012.
Article7. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001; 414:813–820. PMID: 11742414.
Article8. Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes. 2005; 54:1–7. PMID: 15616004.9. Standl E, Baumgartl HJ, Füchtenbusch M, Stemplinger J. Effect of acarbose on additional insulin therapy in type 2 diabetic patients with late failure of sulphonylurea therapy. Diabetes Obes Metab. 1999; 1:215–220. PMID: 11228756.
Article10. Bressler R, Johnson D. New pharmacological approaches to therapy of NIDDM. Diabetes Care. 1992; 15:792–805. PMID: 1600838.
Article11. Kumar S, Narwal S, Kumar V, Prakash O. α-Glucosidase inhibitors from plants: a natural approach to treat diabetes. Pharmacogn Rev. 2011; 5:19–29. PMID: 22096315.
Article12. Mai TT, Thu NN, Tien PG, Van Chuyen N. Alpha-glucosidase inhibitory and antioxidant activities of Vietnamese edible plants and their relationships with polyphenol contents. J Nutr Sci Vitaminol (Tokyo). 2007; 53:267–276. PMID: 17874833.
Article13. Pei R, Yu M, Bruno R, Bolling BW. Phenolic and tocopherol content of autumn olive (Elaeagnus umbellate) berries. J Funct Foods. 2015; 16:305–314.14. Khattak KF. Free radical scavenging activity, phytochemical composition and nutrient analysis of Elaeagnus umbellata berry. J Med Plants Res. 2012; 6:5196–5203.15. Fordham IM, Clevidence BA, Wiley ER, Zimmerman RH. Fruit of autumn olive: a rich source of lycopene. HortScience. 2001; 36:1136–1137.
Article16. Wang SY, Fordham IM. Differences in chemical composition and antioxidant capacity among different genotypes of autumn olive (Elaeagnus umbellate Thunb.). Food Technol Biotechnol. 2007; 45:402–409.17. Meeprom A, Sompong W, Suwannaphet W, Yibchok-anun S, Adisakwattana S. Grape seed extract supplementation prevents high-fructose diet-induced insulin resistance in rats by improving insulin and adiponectin signalling pathways. Br J Nutr. 2011; 106:1173–1181. PMID: 21736810.
Article18. Watanabe J, Kawabata J, Kurihara H, Niki R. Isolation and identification of α-glucosidase inhibitors from tochu-cha (Eucommia ulmoides). Biosci Biotechnol Biochem. 1997; 61:177–178. PMID: 9028049.19. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993; 123:1939–1951. PMID: 8229312.
Article20. Haffner SM, Miettinen H, Stern MP. The homeostasis model in the San Antonio Heart Study. Diabetes Care. 1997; 20:1087–1092. PMID: 9203442.
Article21. Heo SJ, Hwang JY, Choi JI, Han JS, Kim HJ, Jeon YJ. Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown algae, a potent α-glucosidase and α-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Eur J Pharmacol. 2009; 615:252–256. PMID: 19482018.22. Karato M, Yamaguchi K, Takei S, Kino T, Yazawa K. Inhibitory effects of pasuchaca (Geranium dielsiaum) extract on α-glucosidase in mouse. Biosci Biotechnol Biochem. 2006; 70:1482–1484. PMID: 16794329.23. Miura T, Koide T, Ohichi R, Kako M, Usami M, Ishihara E, Yasuda N, Ishida H, Seino Y, Tanigawa K. Effect of acarbose (α-glucosidase inhibitor) on disaccharase activity in small intestine in KK-Ay and ddY mice. J Nutr Sci Vitaminol (Tokyo). 1998; 44:371–379. PMID: 9742458.24. Gerich JE. Clinical significance, pathogenesis, and management of postprandial hyperglycemia. Arch Intern Med. 2003; 163:1306–1316. PMID: 12796066.
Article25. Bahadoran Z, Mirmiran P, Azizi F. Dietary polyphenols as potential nutraceuticals in management of diabetes: a review. J Diabetes Metab Disord. 2013; 12:43. PMID: 23938049.
Article26. Srinivasan K, Ramarao P. Animal models in type 2 diabetes research: an overview. Indian J Med Res. 2007; 125:451–472. PMID: 17496368.27. Chiasson JL, Josse RG, Hunt JA, Palmason C, Rodger NW, Ross SA, Ryan EA, Tan MH, Wolever TM. The efficacy of acarbose in the treatment of patients with non-insulin-dependent diabetes mellitus. A multicenter controlled clinical trial. Ann Intern Med. 1994; 121:928–935. PMID: 7734015.
Article28. Holman RR, Cull CA, Turner RC. A randomized double-blind trial of acarbose in type 2 diabetes shows improved glycemic control over 3 years (U.K. Prospective Diabetes Study 44). Diabetes Care. 1999; 22:960–964. PMID: 10372249.
Article29. Meneilly GS, Ryan EA, Radziuk J, Lau DC, Yale JF, Morais J, Chiasson JL, Rabasa-Lhoret R, Maheux P, Tessier D, Wolever T, Josse RG, Elahi D. Effect of acarbose on insulin sensitivity in elderly patients with diabetes. Diabetes Care. 2000; 23:1162–1167. PMID: 10937515.
Article30. Breuer HW. Review of acarbose therapeutic strategies in the long-term treatment and in the prevention of type 2 diabetes. Int J Clin Pharmacol Ther. 2003; 41:421–440. PMID: 14703948.
Article31. Nigro E, Scudiero O, Monaco ML, Palmieri A, Mazzarella G, Costagliola C, Bianco A, Daniele A. New insight into adiponectin role in obesity and obesity-related diseases. BioMed Res Int. 2014; 2014:658913. PMID: 25110685.
Article32. Alexandraki KI, Piperi C, Ziakas PD, Apostolopoulos NV, Makrilakis K, Syriou V, Diamanti-Kandarakis E, Kaltsas G, Kalofoutis A. Cytokine secretion in long-standing diabetes mellitus type 1 and 2: associations with low-grade systemic inflammation. J Clin Immunol. 2008; 28:314–321. PMID: 18224429.
Article33. Navarro JF, Mora C. Role of inflammation in diabetic complications. Nephrol Dial Transplant. 2005; 20:2601–2604. PMID: 16188894.
Article34. Polyzos SA, Kountouras J, Zavos C, Tsiaousi E. The role of adiponectin in the pathogenesis and treatment of non-alcoholic fatty liver disease. Diabetes Obes Metab. 2010; 12:365–383. PMID: 20415685.
Article35. Monnier L, Colette C, Monnier L, Colette C. Contributions of fasting and postprandial glucose to hemoglobin A1c. Endocr Pract. 2006; 12(Suppl 1):42–46. PMID: 16627379.
Article36. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002; 346:393–403. PMID: 11832527.
Article37. O'Keefe JH Jr, Miles JM, Harris WH, Moe RM, McCallister BD. Improving the adverse cardiovascular prognosis of type 2 diabetes. Mayo Clin Proc. 1999; 74:171–180. PMID: 10069357.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of autumn olive berry extract on insulin resistance and non-alcoholic fatty liver in high fructose-fed rat
- Effects of autumn olive berry on insulin resistance and hyperglycemia in mice fed a high-fat, high-sucrose diet
- A Case of Isolated Glycosuria Mediated by an SLC5A2 Gene Mutation and Characterized by Postprandial Heavy Glycosuria Without Salt Wasting
- The Effects of Postprandial Hyperglycemia on Glucose Control
- Measurement and Treatment Goal of Postprandial Hyperglycemia