Korean J Hepatobiliary Pancreat Surg.
2013 May;17(2):53-59. 10.14701/kjhbps.2013.17.2.53.
Hepatogenic differentiation of human mesenchymal stem cells from peritoneal adipose tissue
- Affiliations
-
- 1Department of Surgery, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 2Department of Surgery, Bucheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Bucheon, Korea. parkiy@catholic.ac.kr
- KMID: 1429979
- DOI: http://doi.org/10.14701/kjhbps.2013.17.2.53
Abstract
- BACKGROUNDS/AIMS
It has been reported that functional hepatogenic differentiation has the possibility to occur in subcutaneous adipose tissue-derived stem cells. However, no studies have investigated whether the adipose tissue-driven stem cells present in various body parts differ according to hepatogenic differentiations. In this study, stem cells were separated from body visceral fat and abdominal subcutaneous adipose tissue, and cultured, and then hepatogenic differentiation was induced. We aim to investigate the possibilities and aspects of hepatogenic differentiations within the two types of fat cells.
METHODS
Omental fat tissues were obtained as visceral fat and abdominal subcutaneous adipose tissues were obtained from patients who had suction-assisted lipectomy. Stem cells were separated from the obtained fat tissues, and then, hepatogenic differentiation was carried out by utilizing 2-step differentiation protocols.
RESULTS
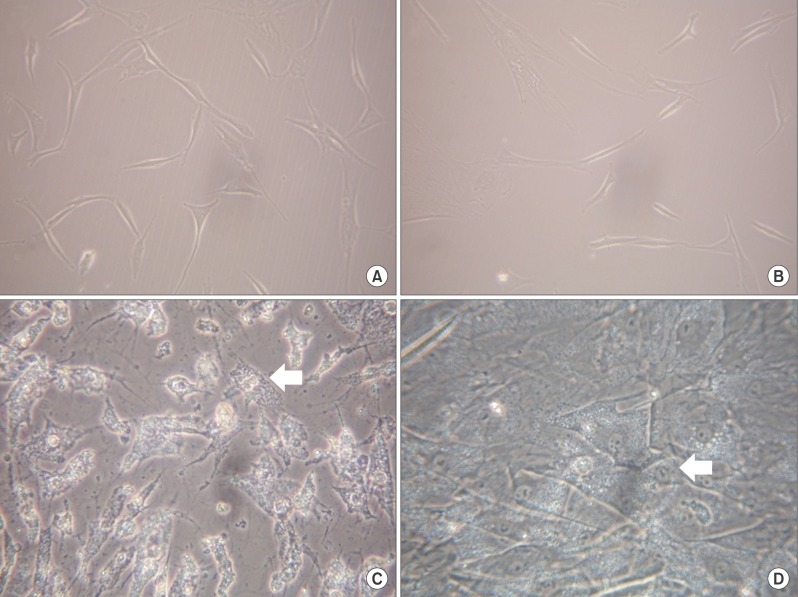
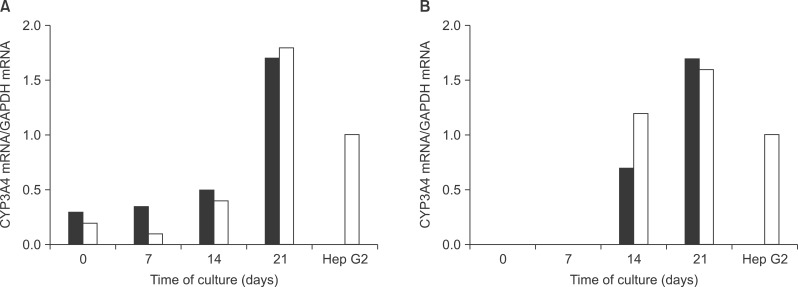
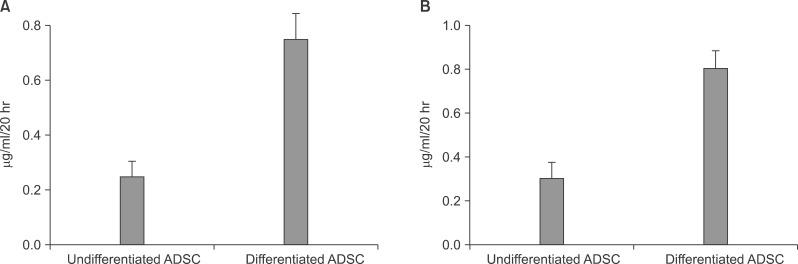
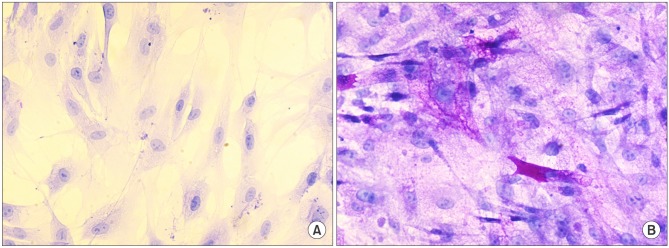
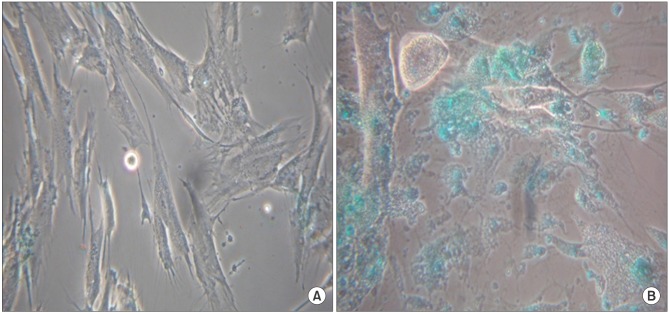
After the differentiation, two types of cultured cells that showed the similar neuron-like shapes were changed to cuboidal shapes and included several binucleated cells which could be characteristics of mature hepatocytes. We confirmed that hepatocyte specific genes and proteins such as albumin and CYP3A4 were being expressed. By utilizing the ELISA test, we were able to observe that the albumin was secreted into the culture fluids in both cells. After completing the differentiation, we observed the presence of the hepatocyte specific properties by confirming glycogen storage within the cells and the ICG reagent uptake.
CONCLUSIONS
We confirmed that hepatogenic differentiation was possible to occur in the omental fat as well as subcutaneous adipose tissue.
MeSH Terms
Figure
Reference
-
1. Aurich I, Mueller LP, Aurich H, et al. Functional integration of hepatocytes derived from human mesenchymal stem cells into mouse livers. Gut. 2007; 56:405–415. PMID: 16928726.
Article2. Aurich H, Sgodda M, Kaltwasser P, et al. Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut. 2009; 58:570–581. PMID: 19022918.
Article3. Banas A, Teratani T, Yamamoto Y, et al. Rapid hepatic fate specification of adipose-derived stem cells and their therapeutic potential for liver failure. J Gastroenterol Hepatol. 2009; 24:70–77. PMID: 18624899.
Article4. Rodriguez AM, Elabd C, Amri EZ, et al. The human adipose tissue is a source of multipotent stem cells. Biochimie. 2005; 87:125–128. PMID: 15733747.
Article5. Rooney GE, Nistor GI, Barry FB, et al. In vitro differentiation potential of human embryonic versus adult stem cells. Regen Med. 2010; 5:365–379. PMID: 20455648.6. Young HE, Mancini ML, Wright RP, et al. Mesenchymal stem cells reside within the connective tissues of many organs. Dev Dyn. 1995; 202:137–144. PMID: 7734732.
Article7. Meliga E, Strem BM, Duckers HJ, et al. Adipose-derived cells. Cell Transplant. 2007; 16:963–970. PMID: 18293895.
Article8. Nakagami H, Morishita R, Maeda K, et al. Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J Atheroscler Thromb. 2006; 13:77–81. PMID: 16733294.
Article9. Banas A, Teratani T, Yamamoto Y, et al. IFATS collection: in vivo therapeutic potential of human adipose tissue mesenchymal stem cells after transplantation into mice with liver injury. Stem Cells. 2008; 26:2705–2712. PMID: 18535155.
Article10. Coradeghini R, Guida C, Scanarotti C, et al. A comparative study of proliferation and hepatic differentiation of human adipose-derived stem cells. Cells Tissues Organs. 2010; 191:466–477. PMID: 20051678.
Article11. Seo MJ, Suh SY, Bae YC, et al. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005; 328:258–264. PMID: 15670778.
Article12. Taléns-Visconti R, Bonora A, Jover R, et al. Hepatogenic differentiation of human mesenchymal stem cells from adipose tissue in comparison with bone marrow mesenchymal stem cells. World J Gastroenterol. 2006; 12:5834–5845. PMID: 17007050.
Article13. Lee KD, Kuo TK, Whang-Peng J, et al. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004; 40:1275–1284. PMID: 15562440.
Article14. Banas A, Teratani T, Yamamoto Y, et al. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007; 46:219–228. PMID: 17596885.
Article15. Sen A, Lea-Currie YR, Sujkowska D, et al. Adipogenic potential of human adipose derived stromal cells from multiple donors is heterogeneous. J Cell Biochem. 2001; 81:312–319. PMID: 11241671.
Article16. Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001; 7:211–228. PMID: 11304456.
Article17. Xu Y, Malladi P, Wagner DR, et al. Adipose-derived mesenchymal cells as a potential cell source for skeletal regeneration. Curr Opin Mol Ther. 2005; 7:300–305. PMID: 16121695.18. Wosnitza M, Hemmrich K, Groger A, et al. Plasticity of human adipose stem cells to perform adipogenic and endothelial differentiation. Differentiation. 2007; 75:12–23. PMID: 17244018.
Article19. Safford KM, Hicok KC, Safford SD, et al. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun. 2002; 294:371–379. PMID: 12051722.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adipose-derived stem cells: characterization and clinical application
- Adipose Tissue Derived Mesenchymal Stem Cells
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Bone Regeneration from Adipose Stem Cells
- Comparison with human amniotic membrane- and adipose tissue-derived mesenchymal stem cells