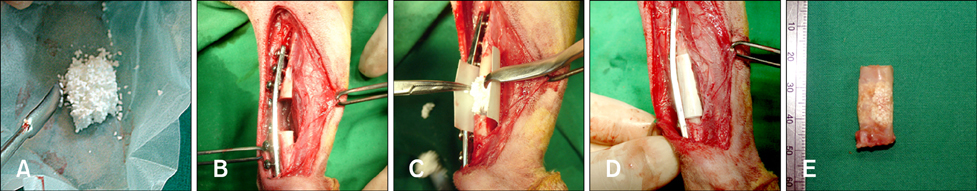
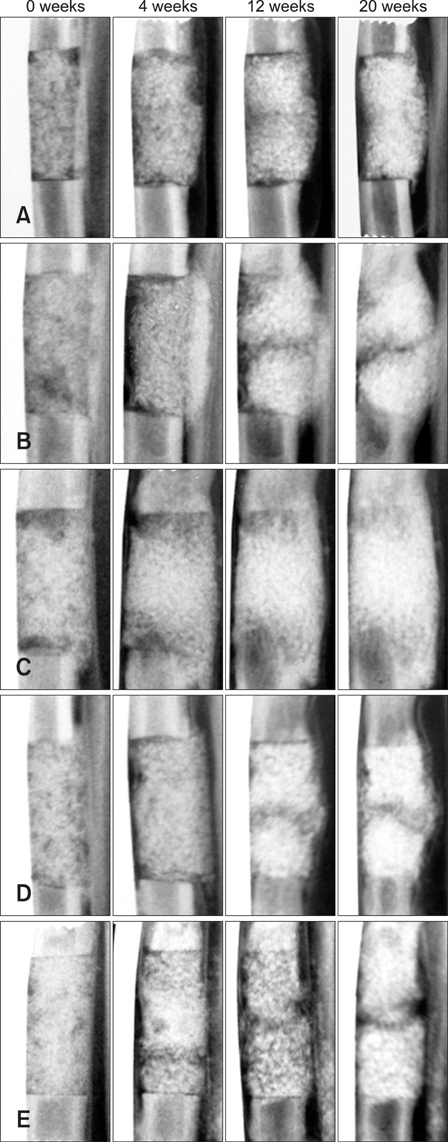
Comparing the osteogenic potential of canine mesenchymal stem cells derived from adipose tissues, bone marrow, umbilical cord blood, and Wharton's jelly for treating bone defects
- Affiliations
-
- 1Department of Veterinary Surgery, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. ohkweon@snu.ac.kr
- 2Biomaterials Center, National Institute for Materials Science, Ibaraki 305-0044, Japan.
- 3College of Veterinary Medicine and KNU Stem Cell Institute, Kangwon National University, Chuncheon 200-701, Korea.
- KMID: 1389770
- DOI: http://doi.org/10.4142/jvs.2012.13.3.299
Abstract
- Alternative sources of mesenchymal stem cells (MSCs) for replacing bone marrow (BM) have been extensively investigated in the field of bone tissue engineering. The purpose of this study was to compare the osteogenic potential of canine MSCs derived from adipose tissue (AT), BM, umbilical cord blood (UCB), and Wharton's jelly (WJ) using in vitro culture techniques and in vivo orthotopic implantation assays. After canine MSCs were isolated from various tissues, the proliferation and osteogenic potential along with vascular endothelial growth factor (VEGF) production were measured and compared in vitro. For the in vivo assay, MSCs derived from each type of tissue were mixed with beta-tricalcium phosphate and implanted into segmental bone defects in dogs. Among the different types of MSCs, AT-MSCs had a higher proliferation potential and BM-MSCs produced the most VEGF. AT-MSCs and UCB-MSCs showed greater in vitro osteogenic potential compared to the other cells. Radiographic and histological analyses showed that all tested MSCs had similar osteogenic capacities, and the level of new bone formation was much higher with implants containing MSCs than cell-free implants. These results indicate that AT-MSCs, UCB-MSCs, and WJ-MSCs can potentially be used in place of BM-MSCs for clinical bone engineering procedures.
Keyword
MeSH Terms
-
Adipocytes, White/cytology/physiology
Alkaline Phosphatase/metabolism
Animals
Biocompatible Materials/metabolism/*therapeutic use
Bone Diseases/*therapy
Bone Marrow Cells/cytology/physiology
Calcification, Physiologic
Calcium/metabolism
Calcium Phosphates/metabolism/therapeutic use
Cell Proliferation
Dogs
Female
Fetal Blood/cytology/physiology
Flow Cytometry
Male
Mesenchymal Stromal Cells/cytology/*metabolism
*Osteogenesis
Polyesters/metabolism/therapeutic use
Tissue Engineering/*methods
Vascular Endothelial Growth Factor A/metabolism
Figure
Cited by 3 articles
-
Effect of serum-derived albumin scaffold and canine adipose tissue-derived mesenchymal stem cells on osteogenesis in canine segmental bone defect model
Daeyoung Yoon, Byung-Jae Kang, Yongsun Kim, Seung Hoon Lee, Daeun Rhew, Wan Hee Kim, Oh-Kyeong Kweon
J Vet Sci. 2015;16(4):397-404. doi: 10.4142/jvs.2015.16.4.397.Extensive characterization of feline intra-abdominal adipose-derived mesenchymal stem cells
Hee-Ryang Kim, Jienny Lee, Jeong Su Byeon, Na-Yeon Gu, Jiyun Lee, In-Soo Cho, Sang-Ho Cha
J Vet Sci. 2017;18(3):299-306. doi: 10.4142/jvs.2017.18.3.299.Comparison of the characteristics of canine adipose tissue-derived mesenchymal stem cells extracted from different sites and at different passage numbers
Kevin M. Yaneselli, Cristiana P. Kuhl, Paula B. Terraciano, Fernanda S. de Oliveira, Sabrina B. Pizzato, Kamila Pazza, Alessandra B. Magrisso, Vanessa Torman, Analía Rial, María Moreno, Silvia Llambí, Elizabeth Cirne-Lima, Jacqueline Maisonnave
J Vet Sci. 2018;19(1):13-20. doi: 10.4142/jvs.2018.19.1.13.
Reference
-
1. Al-Khaldi A, Eliopoulos N, Martineau D, Lejeune L, Lachapelle K, Galipeau J. Postnatal bone marrow stromal cells elicit a potent VEGF-dependent neoangiogenic response in vivo. Gene Ther. 2003. 10:621–629.
Article2. Arinzeh TL, Peter SJ, Archambault MP, van den Bos C, Gordon S, Kraus K, Smith A, Kadiyala S. Allogeneic mesenchymal stem cells regenerate bone in a critical-sized canine segmental defect. J Bone Joint Surg Am. 2003. 85:1927–1935.
Article3. Arthur A, Zannettino A, Gronthos S. The therapeutic applications of multipotential mesenchymal/stromal stem cells in skeletal tissue repair. J Cell Physiol. 2009. 218:237–245.
Article4. Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002. 30:42–48.
Article5. Bianco P, Kuznetsov SA, Riminucci M, Robey PG. Postnatal skeletal stem cells. Methods Enzymol. 2006. 419:117–148.
Article6. Bruder SP, Kraus KH, Goldberg VM, Kadiyala S. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J Bone Joint Surg Am. 1998. 80:985–996.
Article7. Byeon YE, Ryu HH, Park SS, Koyama Y, Kikuchi M, Kim WH, Kang KS, Kweon OK. Paracrine effect of canine allogenic umbilical cord blood-derived mesenchymal stromal cells mixed with beta-tricalcium phosphate on bone regeneration in ectopic implantations. Cytotherapy. 2010. 12:626–636.
Article8. Cancedda R, Giannoni P, Mastrogiacomo M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials. 2007. 28:4240–4250.
Article9. Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001. 7:259–264.
Article10. Chang YJ, Shih DT, Tseng CP, Hsieh TB, Lee DC, Hwang SM. Disparate mesenchyme-lineage tendencies in mesenchymal stem cells from human bone marrow and umbilical cord blood. Stem Cells. 2006. 24:679–685.
Article11. Cho W, Nam S, Jang J, Lee E, Lee E, Son Y. Comparative evaluation of differentiation potentials of various stem cells from mesenchymal tissue origin. Tissue Eng Regen Med. 2010. 7:355–361.12. Ciapetti G, Ambrosio L, Marletta G, Baldini N, Giunti A. Human bone marrow stromal cells: in vitro expansion and differentiation for bone engineering. Biomaterials. 2006. 27:6150–6160.
Article13. Dragoo JL, Samimi B, Zhu M, Hame SL, Thomas BJ, Lieberman JR, Hedrick MH, Benhaim P. Tissue-engineered cartilage and bone using stem cells from human infrapatellar fat pads. J Bone Joint Surg Br. 2003. 85:740–747.
Article14. Drosse I, Volkmer E, Capanna R, De Biase P, Mutschler W, Schieker M. Tissue engineering for bone defect healing: an update on a multi-component approach. Injury. 2008. 39:Suppl 2. S9–S20.
Article15. Gauthaman K, Venugopal JR, Yee FC, Biswas A, Ramakrishna S, Bongso A. Osteogenic differentiation of human Wharton's jelly stem cells on nanofibrous substrates in vitro. Tissue Eng Part A. 2011. 17:71–81.
Article16. Gregory CA, Gunn WG, Peister A, Prockop DJ. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004. 329:77–84.
Article17. Haque MA, Nagaoka M, Hexig B, Akaike T. Artificial extracellular matrix for embryonic stem cell cultures: a new frontier of nanobiomaterials. Sci Technol Adv Mat. 2010. 11:014106.
Article18. Hattori H, Masuoka K, Sato M, Ishihara M, Asazuma T, Takase B, Kikuchi M, Nemoto K, Ishihara M. Bone formation using human adipose tissue-derived stromal cells and a biodegradable scaffold. J Biomed Mater Res B Appl Biomater. 2006. 76:230–239.
Article19. Hayashi O, Katsube Y, Hirose M, Ohgushi H, Ito H. Comparison of osteogenic ability of rat mesenchymal stem cells from bone marrow, periosteum, and adipose tissue. Calcif Tissue Int. 2008. 82:238–247.
Article20. Janicki P, Kasten P, Kleinschmidt K, Luginbuehl R, Richter W. Chondrogenic pre-induction of human mesenchymal stem cells on beta-TCP: enhanced bone quality by endochondral heterotopic bone formation. Acta Biomater. 2010. 6:3292–3301.
Article21. Kaigler D, Krebsbach PH, Polverini PJ, Mooney DJ. Role of vascular endothelial growth factor in bone marrow stromal cell modulation of endothelial cells. Tissue Eng. 2003. 9:95–103.
Article22. Kasten P, Vogel J, Luginbühl R, Niemeyer P, Tonak M, Lorenz H, Helbig L, Weiss S, Fellenberg J, Leo A, Simank HG, Richter W. Ectopic bone formation associated with mesenchymal stem cells in a resorbable calcium deficient hydroxyapatite carrier. Biomaterials. 2005. 26:5879–5889.
Article23. Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006. 24:1294–1301.
Article24. Kikuchi M, Koyama Y, Yamada T, Imamura Y, Okada T, Shirahama N, Akita K, Takakuda K, Tanaka J. Development of guided bone regeneration membrane composed of β-tricalcium phosphate and poly (L-lactide-co-glycolide-co-ε-caprolactone) composites. Biomaterials. 2004. 25:5979–5986.
Article25. Kon E, Muraglia A, Corsi A, Bianco P, Marcacci M, Martin I, Boyde A, Ruspantini I, Chistolini P, Rocca M, Giardino R, Cancedda R, Quarto R. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J Biomed Mater Res. 2000. 49:328–337.
Article26. Kruyt MC, Dhert WJA, Oner FC, van Blitterswijk CA, Verbout AJ, de Bruijn JD. Analysis of ectopic and orthotopic bone formation in cell-based tissue-engineered constructs in goats. Biomaterials. 2007. 28:1798–1805.
Article27. Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004. 103:1669–1675.
Article28. Mastrogiacomo M, Corsi A, Francioso E, Di Comite M, Monetti F, Scaglione S, Favia A, Crovace A, Bianco P, Cancedda R. Reconstruction of extensive long bone defects in sheep using resorbable bioceramics based on silicon stabilized tricalcium phosphate. Tissue Eng. 2006. 12:1261–1273.
Article29. Petite H, Viateau V, Bensaïd W, Meunier A, de Pollak C, Bourguignon M, Oudina K, Sedel L, Guillemin G. Tissue-engineered bone regeneration. Nat Biotechnol. 2000. 18:959–963.
Article30. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.
Article31. Rauch C, Brunet AC, Deleule J, Farge E. C2C12 myoblast/osteoblast transdifferentiation steps enhanced by epigenetic inhibition of BMP2 endocytosis. Am J Physiol Cell Physiol. 2002. 283:C235–C243.32. Schäffler A, Büchler C. Concise review: adipose tissue-derived stromal cells--basic and clinical implications for novel cell-based therapies. Stem Cells. 2007. 25:818–827.
Article33. Woodbury D, Reynolds K, Black IB. Adult bone marrow stromal stem cells express germline, ectodermal, endodermal, and mesodermal genes prior to neurogenesis. J Neurosci Res. 2002. 69:908–917.
Article34. Yang F, Cho SW, Son SM, Hudson SP, Bogatyrev S, Keung L, Kohane DS, Langer R, Anderson DG. Combinatorial extracellular matrices for human embryonic stem cell differentiation in 3D. Biomacromolecules. 2010. 11:1909–1914.
Article35. Yuan J, Cui L, Zhang WJ, Liu W, Cao Y. Repair of canine mandibular bone defects with bone marrow stromal cells and porous β-tricalcium phosphate. Biomaterials. 2007. 28:1005–1013.
Article36. Zhang X, Xie C, Lin ASP, Ito H, Awad H, Lieberman JR, Rubery PT, Schwarz EM, O'Keefe RJ, Guldberg RE. Periosteal progenitor cell fate in segmental cortical bone graft transplantations: implications for functional tissue engineering. J Bone Miner Res. 2005. 20:2124–2137.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential Potential of Stem Cells Following Their Origin: Subacromial Bursa, Bone Marrow, Umbilical Cord Blood
- Osteogenic potential of mesenchymal cells derived from canine umbilical cord matrix co-cultured with platelet-rich plasma and demineralized bone matrix
- Clinical Use of Mesenchymal Stem Cells in Bone Regeneration
- Use of Cord Blood Stem Cells in Cell Therapy
- Difference in HLA-DR Expression of Human Umbilical Cord Blood Derived Mesenchymal Stem Cells after Tri-lineage Differentiation