J Korean Med Sci.
2012 May;27(5):547-552. 10.3346/jkms.2012.27.5.547.
Hepatic Ischemic Preconditioning Provides Protection Against Distant Renal Ischemia and Reperfusion Injury in Mice
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Saint Vincent Hospital, The Catholic University of Korea College of Medicine, Suwon, Korea. joojd@catholic.ac.krz
- KMID: 1372799
- DOI: http://doi.org/10.3346/jkms.2012.27.5.547
Abstract
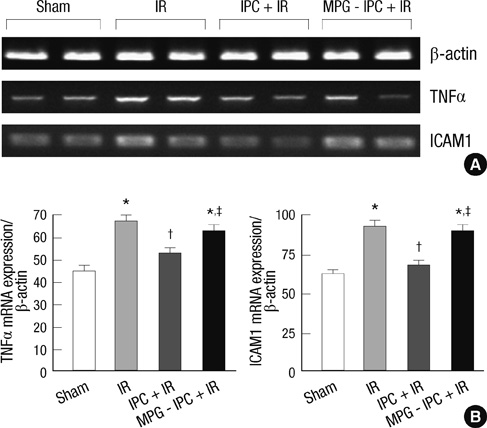
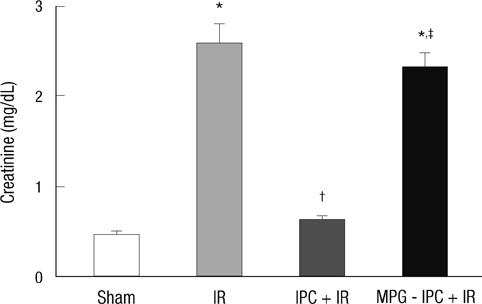
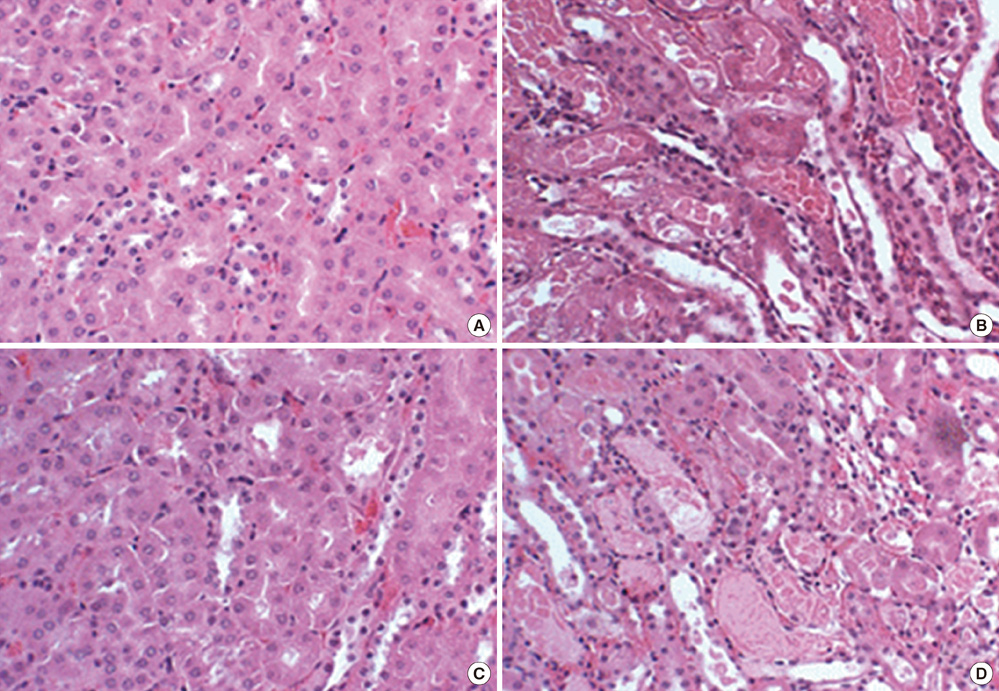
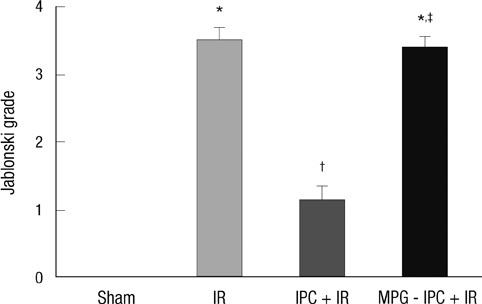
- We previously demonstrated that there are acute and delayed phases of renal protection against renal ischemia and reperfusion (IR) injury with renal ischemic preconditioning (IPC). This study assessed whether hepatic IPC could also reduce distant renal IR injury through the blood stream-mediated supply of reactive oxygen species (ROS). Male C57BL/6 mice were randomly divided into four groups: group I, sham operated including right nephrectomy; group II (IR), left renal ischemia for 30 min and reperfusion injury; group III (IPC-IR), hepatic ischemia for 10 min followed by 10 min of reperfusion before left renal IR injury; group IV (MPG - IPC + IR), pretreated with 100 mg/kg N-(2-mercaptopropionyl)-glycine (MPG) 15 min before hepatic IPC and left renal IR injury. Renal function, histopathologic findings, proinflammatory cytokines, and cytoprotective proteins were evaluated 15 min or 24 hr after reperfusion. Hepatic IPC attenuated the expression of proinflammatory cytokines, tumor necrosis factor alpha, intercellular adhesion molecule 1, and induced inducible nitric-oxide synthase, and the phosphorylation of Akt in the murine kidney. Renal function was better preserved in mice with hepatic IPC (group III) than groups II or IV. Hepatic IPC protects against distant renal IR injury through the blood stream-delivery of hepatic IPC-induced ROS, by inducing cytoprotective proteins, and by inhibiting inflammatory reactions.
Keyword
MeSH Terms
-
Animals
Intercellular Adhesion Molecule-1/genetics/metabolism
*Ischemic Preconditioning
Kidney/drug effects/metabolism/pathology/physiopathology
Liver/blood supply/drug effects/physiopathology
Male
Mice
Mice, Inbred C57BL
Nitric Oxide Synthase Type II/metabolism
Phosphorylation
Proto-Oncogene Proteins c-akt/metabolism
Reactive Oxygen Species/metabolism
Reperfusion Injury/*metabolism/pathology/prevention & control
Tiopronin/pharmacology
Tumor Necrosis Factor-alpha/genetics/metabolism
Figure
Reference
-
1. Joo JD, Kim M, Horst P, Kim J, D'Agati VD, Emala CW Sr, Lee HT. Acute and delayed renal protection against renal ischemia and reperfusion injury with A1 adenosine receptors. Am J Physiol Renal Physiol. 2007. 293:F1847–F1857.2. Ridao-Cano N, Rodriguez A, Torrente J, Gallego J, Barrientos A. Acute renal failure following endovascular repair of an infrarenal abdominal aortic aneurysm. Nephrol Dial transplant. 2006. 21:221–222.3. Izuishi K, Tsung A, Hossain MA, Fujiwara M, Wakabayashi H, Masaki T, Billiar TR, Maeta H. Ischemic preconditioning of the murine liver protects through the Akt kinase pathway. Hepatology. 2006. 44:573–580.4. Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003. 284:G15–G26.5. Park SW, Chen SW, Kim M, Brown KM, D'Agati VD, Lee HT. Protection against acute kidney injury via A(1) adenosine receptor-mediated Akt activation reduces liver injury after liver ischemia and reperfusion in mice. J Pharmacol Exp Ther. 2010. 333:736–747.6. Yin W, Signore AP, Iwai M, Cao G, Gao Y, Johnnides MJ, Hickey RW, Chen J. Preconditioning suppresses inflammation in neonatal hypoxic ischemia via Akt activation. Stroke. 2007. 38:1017–1024.7. Joo JD, Kim M, D'Agati VD, Lee HT. Ischemic preconditioning provides both acute and delayed protection against renal ischemia and reperfusion injury in mice. J Am Soc Nephrol. 2006. 17:3115–3123.8. Gallos G, Jones DR, Nasr SH, Emala CW, Lee HT. Local anesthetics reduce mortality and protect against renal and hepatic dysfunction in murine septic peritonitis. Anesthesiology. 2004. 101:902–911.9. Joo JD, Choi JW, In JH, Jung HS, Lee JA, Kim YS, Kim DW, Yeom JH, Shin EY, Jeon YS. Lidocaine suppresses the increased extracellular signal-regulated kinase/cyclic AMP response element-binding protein pathway and pro-inflammatory cytokines in a neuropathic pain model of rats. Eur J Anaesthesiol. 2011. 28:106–111.10. Lee HT, Ota-Setlik A, Fu Y, Nasr SH, Emala CW. Differential protective effects of volatile anesthetics against renal ischemia-reperfusion injury in vivo. Anesthesiology. 2004. 101:1313–1324.11. Jablonski P, Howden BO, Rae DA, Birrell CS, Marshall VC, Tange J. An experimental model for assessment of renal recovery from warm ischemia. Transplantation. 1983. 35:198–204.12. Ateş E, Genç E, Erkasap N, Erkasap S, Akman S, Firat P, Emre S, Kiper H. Renal protection by brief liver ischemia in rats. Transplantation. 2002. 74:1247–1251.13. Tapuria N, Kumar Y, Habib MM, Abu Amara M, Seifalian AM, Davidson BR. Remote ischemic preconditioning: a novel protective method from ischemia reperfusion injury: a review. J Surg Res. 2008. 150:304–330.14. Joo JD, Kim DW, Kang YJ, Kim YS, Jeon YS, In JH, Choi JW, Park YJ. Renal protective effects of opposite renal ischemic preconditioning against renal ischemic reperfusion injury in mice. Korean J Anesthesiol. 2007. 53:229–233.15. Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev. 2007. 12:181–188.16. Alchera E, Dal Ponte C, Imarisio C, Albano E, Carini R. Molecular mechanisms of liver preconditioning. World J Gastroenterol. 2010. 16:6058–6067.17. Yamashita J, Ogata M, Itoh M, Yamasowa H, Shimeda Y, Takaoka M, Matsumura Y. Role of nitric oxide in the renal protective effects of ischemic preconditioning. J Cardiovasc Pharmacol. 2003. 42:419–427.18. Rhee JE, Jung SE, Shin SD, Suh GJ, Noh DY, Youn YK, Oh SK, Choe KJ. The effects of antioxidants and nitric oxide modulators on hepatic ischemic-reperfusion injury in rats. J Korean Med Sci. 2002. 17:502–506.19. Imagawa J, Yellon DM, Baxter GF. Pharmacological evidence that inducible nitric oxide synthase is a mediator of delayed preconditioning. Br J Pharmacol. 1999. 126:701–708.20. Baines CP, Goto M, Downey JM. Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J Mol Cell Cardiol. 1997. 29:207–216.21. Zhang W, Wang M, Xie HY, Zhou L, Meng XQ, Shi J, Zheng S. Role of reactive oxygen species in mediating hepatic ischemia-reperfusion injury and its therapeutic applications in liver transplantation. Transplant Proc. 2007. 39:1332–1337.22. Torres M, Forman HJ. Redox signaling and the MAP kinase pathways. Biofactors. 2003. 17:287–296.23. Koti RS, Tsui J, Lobos E, Yang W, Seifalian AM, Davidson BR. Nitric oxide synthase distribution and expression with ischemic preconditioning of the rat liver. FASEB J. 2005. 19:1155–1157.24. Kaysen GA, Eiserich JP. Characteristics and effects of inflammation in end-stage renal disease. Semin Dial. 2003. 16:438–446.25. Seal JB, Gewertz BL. Vascular dysfunction in ischemia-reperfusion injury. Ann Vasc Surg. 2005. 19:572–584.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Renal Protective Effects of Opposite Renal Ischemic Preconditioning against Renal Ischemic Reperfusion Injury in Mice
- The Effect of Hypoxic-Preconditioning on the Reperfusion-Induced Arrhythmias in the Cat Hearts
- Effect of Ischemic Preconditioning on Catecholamine Release from the Isolated, Ischemic Reperfused Hearts of Rats
- Exploration of the interaction between remote ischemic preconditioning and anesthetic-induced preconditioning using sevoflurane in isolated perfused rabbit heart
- Alteration of NF -kappa kappaB in Ischemic -reperfused Anterior Tibialis and Soleus Muscles of Rats