Yonsei Med J.
2007 Jun;48(3):526-530. 10.3349/ymj.2007.48.3.526.
Initiation Site of Ca2+ Entry Evoked by Endoplasmic Reticulum Ca2+ Depletion in Mouse Parotid and Pancreatic Acinar Cells
- Affiliations
-
- 1Yonsei University College of Dentistry, 250 Seongsanno, Seodaemon-gu, Seoul 120-752, Korea. dmshin@yumc.yonsei.ac.kr
- KMID: 724110
- DOI: http://doi.org/10.3349/ymj.2007.48.3.526
Abstract
- PURPOSE
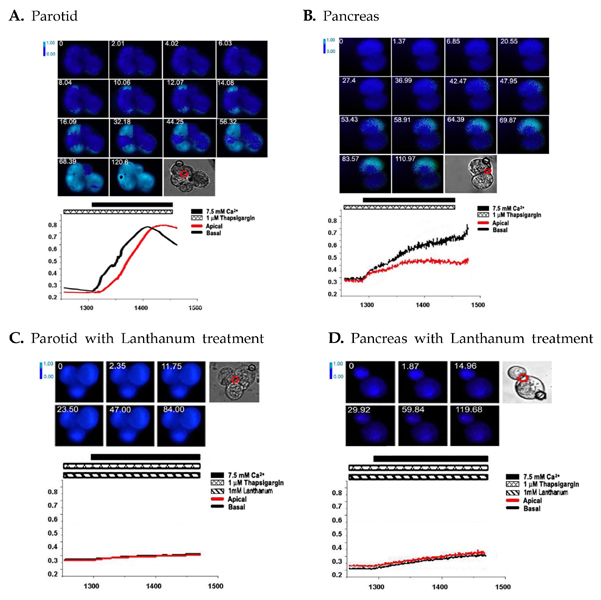
In non-excitable cells, which include parotid and pancreatic acinar cells, Ca(2+) entry is triggered via a mechanism known as capacitative Ca(2+) entry, or store-operated Ca(2+) entry. This process is initiated by the perception of the filling state of endoplasmic reticulum (ER) and the depletion of internal Ca(2+) stores, which acts as an important factor triggering Ca(2+) entry. However, both the mechanism of store-mediated Ca(2+) entry and the molecular identity of store-operated Ca(2+) channel (SOCC) remain uncertain. MATERIALS AND METHODS: In the present study we investigated the Ca(2+) entry initiation site evoked by depletion of ER to identify the localization of SOCC in mouse parotid and pancreatic acinar cells with microfluorometeric imaging system. RESULTS: Treatment with thapsigargin (Tg), an inhibitor of sarco/endoplasmic reticulum Ca(2+)-ATPase, in an extracellular Ca(2+) free state, and subsequent exposure to a high external calcium state evoked Ca(2+) entry, while treatment with lanthanum, a non-specific blocker of plasma Ca(2+) channel, completely blocked Tg-induced Ca(2+) entry. Microfluorometric imaging showed that Tg-induced Ca(2+) entry started at a basal membrane, not a apical membrane. CONCLUSION: These results suggest that Ca2+ entry by depletion of the ER initiates at the basal pole in polarized exocrine cells and may help to characterize the nature of SOCC.
MeSH Terms
Figure
Cited by 1 articles
-
Poorly-Controlled Type 1 Diabetes Mellitus Impairs LH-LHCGR Signaling in the Ovaries and Decreases Female Fertility in Mice
Jaewang Lee, Hoi Chang Lee, So-Youn Kim, Geum Joon Cho, Teresa K. Woodruff
Yonsei Med J. 2019;60(7):667-678. doi: 10.3349/ymj.2019.60.7.667.
Reference
-
1. Missiaen L, Robberecht W, van der Bosch L, Callewaert G, Parys JB, Wuytack F, et al. Abnormal intracellular Ca2+ homeostasis and disease. Cell Calcium. 2000. 28:1–21.2. Berridge MJ. Inositol trisphosphate and calcium signaling. Nature. 1993. 361:315–325.
Article3. Lee HC, Munshi C, Graeff R. Structures and activities of cyclic ADP-ribose, NAADP and their metabolic enzymes. Mol Cell Biochem. 1999. 193:89–98.
Article4. Petersen OH, Cancela JM. New Ca2+-releasing messengers: are they important in the nervous system? Trends Neurosci. 1999. 22:488–495.5. Clementi E, Meldolesi J. Pharmacological and functional properties of voltage-independent Ca2+ channels. Cell Calcium. 1996. 19:269–279.
Article6. Putney JW Jr. A model for receptor-regulated calcium entry. Cell Calcium. 1986. 7:1–12.
Article7. Putney JW Jr. Capacitative Calcium Entry. 1997. Austin, TX: Landes Biomedical Publishing;210.8. Putney JW Jr, Bird GS. The signal for capacitative calcium entry. Cell. 1993. 75:199–201.
Article9. Tsien RW, Tsien RY. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990. 6:715–760.
Article10. Parekh AB, Penner R. Depletion-activated calcium current is inhibited by protein kinase in RBL-2H3 cells. Proc Natl Acad Sci U S A. 1995. 92:7907–7911.
Article11. Lin WW, Chuang DM. Endothelin- and ATP-induced inhibition of adenylyl cyclase activity in C6 glioma cells: role of Gi and calcium. Mol Pharmacol. 1993. 44:158–165.12. Tsunoda Y, Stuenkel EL, Williams JA. Characterization of sustained [Ca2+]i increase in pancreatic acinar cells and its relation to amylase secretion. Am J Physiol. 1990. 259:G792–G801.13. Mertz LM, Horn VJ, Baum BJ, Ambudkar IS. Calcium entry in rat parotid acini: activation by carbachol and aluminum fluoride. Am J Physiol. 1990. 258:C654–C661.
Article14. Zeng W, Lee MG, Yan M, Diaz J, Benjamin I, Marino CR, et al. Immuno and functional characterization of CFTR in submandibular and pancreatic acinar and duct cells. Am J Physiol. 1997. 273:C442–C455.
Article15. Xu X, Diaz J, Zhao H, Muallem S. Characterization, localization and axial distribution of Ca2+ signalling receptors in the rat submandibular salivary gland ducts. J Physiol. 1996. 491:647–662.
Article16. Hong JH, Lee SI, Kim KE, Yong TS, Seo JT, Sohn MH, et al. German cockroach extract activates protease- activated receptor 2 in human airway epithelial cells. J Allergy Clin Immunol. 2004. 113:315–319.
Article17. Shin DM, Dehoff M, Luo X, Kang SH, Tu J, Nayak SK, et al. Homer 2 tunes G protein-coupled receptors stimulus intensity by regulating RGS proteins and PLCbeta GAP activities. J Cell Biol. 2003. 162:293–303.
Article18. Segawa A, Sahara N, Suzuki K, Yamashina S. Acinar structure and membrane regionalization as a prerequisite for exocrine secretion in the rat submandibular gland. J Cell Sci. 1985. 78:67–85.
Article19. Yamamoto-Hino M, Miyawaki A, Segawa A, Adachi E, Yamashina S, Fujimoto T, et al. Apical vesicles bearing inositol 1,4,5-trisphosphate receptors in the Ca2+ initiation site of ductal epithelium of submandibular gland. J Cell Biol. 1998. 141:135–142.
Article20. Raraty M, Ward J, Erdemli G, Vaillant C, Neoptolemos JP, Sutton R, et al. Calcium-dependent enzyme activation and vacuole formation in the apical granular region of pancreatic acinar cells. Proc Natl Acad Sci U S A. 2000. 97:13126–13131.
Article21. Toescu EC, Lawrie AM, Petersen OH, Gallacher DV. Spatial and temporal distribution of agonist-evoked cytoplasmic Ca2+ signals in exocrine acinar cells analysed by digital image microscopy. EMBO J. 1992. 11:1623–1629.
Article22. Nathanson MH, Padfield PJ, O'Sullivan AJ, Burgstahler DV, Jamieson JD. Mechanism of Ca2+ wave propagation in pancreatic acinar cells. J Biol Chem. 1992. 267:18118–18121.23. Kasai H, Li YX, Miyashita Y. Subcellular distribution of Ca2+ release channels underlying Ca2+ waves and oscillations in exocrine pancreas. Cell. 1993. 74:669–677.
Article24. Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005. 169:435–445.
Article25. Feske S, Gwack YS, Prakriya M, Srikanth S, Puppel SH, Tanasa B, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006. 441:179–185.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Regulators of G-Protein Signaling 4 in Ca2+ Signaling in Mouse Pancreatic Acinar Cells
- Caffeine and 2-Aminoethoxydiphenyl Borate (2-APB) Have Different Ability to Inhibit Intracellular Calcium Mobilization in Pancreatic Acinar Cell
- Expression of Ca2+-dependent Synaptotagmin Isoforms in Mouse and Rat Parotid Acinar Cells
- The Effect of Ghrelin on Ca2+ Concentration in Thyroid FRTL-5 Cells
- The Effects of DTBNP on Intracellular Ca2+ Signaling in Cultured Bovine Aortic Endothelial Cells