Korean J Physiol Pharmacol.
2011 Dec;15(6):383-388. 10.4196/kjpp.2011.15.6.383.
Role of Regulators of G-Protein Signaling 4 in Ca2+ Signaling in Mouse Pancreatic Acinar Cells
- Affiliations
-
- 1Department of Oral Biology, Yonsei University College of Dentistry, Seoul 120-752, Korea. dmshin@yuhs.ac
- KMID: 2285425
- DOI: http://doi.org/10.4196/kjpp.2011.15.6.383
Abstract
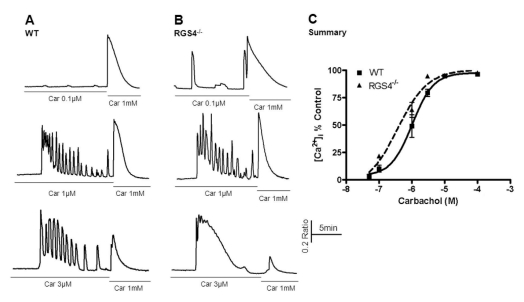
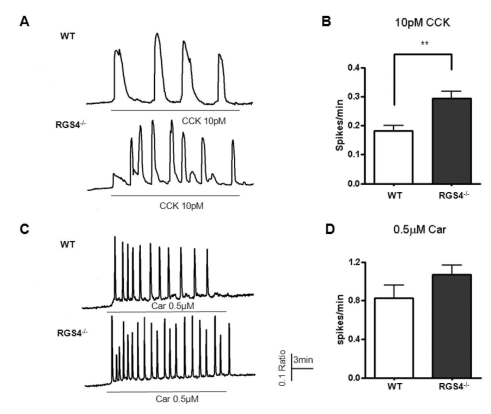
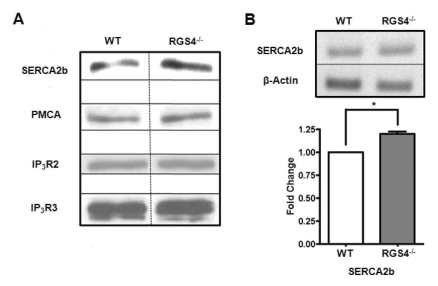
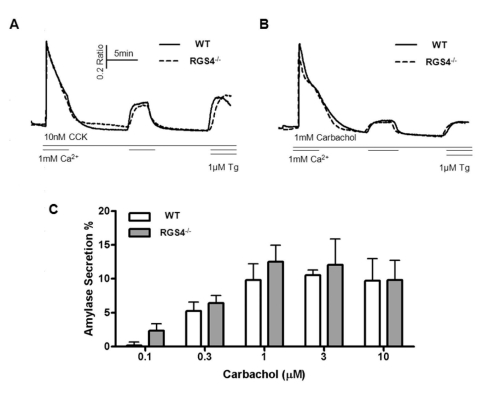
- Regulators of G-protein signaling (RGS) proteins are regulators of Ca2+ signaling that accelerate the GTPase activity of the G-protein alpha-subunit. RGS1, RGS2, RGS4, and RGS16 are expressed in the pancreas, and RGS2 regulates G-protein coupled receptor (GPCR)-induced Ca2+ oscillations. However, the role of RGS4 in Ca2+ signaling in pancreatic acinar cells is unknown. In this study, we investigated the mechanism of GPCR-induced Ca2+ signaling in pancreatic acinar cells derived from RGS4-/- mice. RGS4-/- acinar cells showed an enhanced stimulus intensity response to a muscarinic receptor agonist in pancreatic acinar cells. Moreover, deletion of RGS4 increased the frequency of Ca2+ oscillations. RGS4-/- cells also showed increased expression of sarco/endoplasmic reticulum Ca2+ ATPase type 2. However, there were no significant alterations, such as Ca2+ signaling in treated high dose of agonist and its related amylase secretion activity, in acinar cells from RGS4-/- mice. These results indicate that RGS4 protein regulates Ca2+ signaling in mouse pancreatic acinar cells.
Keyword
MeSH Terms
Figure
Reference
-
1. Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003; 4:517–529. PMID: 12838335.
Article2. Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995; 80:179–185. PMID: 7834738.
Article3. Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000; 1:11–21. PMID: 11413485.
Article4. Kiselyov K, Shin DM, Muallem S. Signalling specificity in GPCR-dependent Ca2+ signalling. Cell Signal. 2003; 15:243–253. PMID: 12531423.5. Mattson MP, Chan SL. Calcium orchestrates apoptosis. Nat Cell Biol. 2003; 5:1041–1043. PMID: 14647298.
Article6. Thorn P, Lawrie AM, Smith PM, Gallacher DV, Petersen OH. Ca2+ oscillations in pancreatic acinar cells: spatiotemporal relationships and functional implications. Cell Calcium. 1993; 14:746–757. PMID: 8131191.7. Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991; 351:751–754. PMID: 1648178.8. Wakui M, Potter BV, Petersen OH. Pulsatile intracellular calcium release does not depend on fluctuations in inositol trisphosphate concentration. Nature. 1989; 339:317–320. PMID: 2498663.
Article9. Willars GB. Mammalian RGS proteins: multifunctional regulators of cellular signalling. Semin Cell Dev Biol. 2006; 17:363–376. PMID: 16687250.
Article10. Chan RK, Otte CA. Physiological characterization of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by a factor and alpha factor pheromones. Mol Cell Biol. 1982; 2:21–29. PMID: 7050666.
Article11. Chan RK, Otte CA. Isolation and genetic analysis of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by a factor and alpha factor pheromones. Mol Cell Biol. 1982; 2:11–20. PMID: 7050665.
Article12. Koelle MR, Horvitz HR. EGL-10 regulates G protein signaling in the C. elegans nervous system and shares a conserved domain with many mammalian proteins. Cell. 1996; 84:115–125. PMID: 8548815.
Article13. De Vries L, Mousli M, Wurmser A, Farquhar MG. GAIP, a protein that specifically interacts with the trimeric G protein G alpha i3, is a member of a protein family with a highly conserved core domain. Proc Natl Acad Sci USA. 1995; 92:11916–11920. PMID: 8524874.
Article14. Berman DM, Wilkie TM, Gilman AG. GAIP and RGS4 are GTPase-activating proteins for the Gi subfamily of G protein alpha subunits. Cell. 1996; 86:445–452. PMID: 8756726.15. Kasai H, Li YX, Miyashita Y. Subcellular distribution of Ca2+ release channels underlying Ca2+ waves and oscillations in exocrine pancreas. Cell. 1993; 74:669–677. PMID: 8395348.16. Thorn P, Lawrie AM, Smith PM, Gallacher DV, Petersen OH. Local and global cytosolic Ca2+ oscillations in exocrine cells evoked by agonists and inositol trisphosphate. Cell. 1993; 74:661–668. PMID: 8395347.17. Choi KJ, Cho DS, Kim JY, Kim BJ, Lee KM, Kim SH, Kim DK, Kim SH, Park HS. Ca2+-induced Ca2+ release from internal stores in INS-1 rat insulinoma cells. Korean J Physiol Pharmacol. 2011; 15:53–59. PMID: 21461241.18. Luo X, Ahn W, Muallem S, Zeng W. Analyses of RGS protein control of agonist-evoked Ca2+ signaling. Methods Enzymol. 2004; 389:119–130. PMID: 15313563.19. Wang X, Huang G, Luo X, Penninger JM, Muallem S. Role of regulator of G protein signaling 2 (RGS2) in Ca2+ oscillations and adaptation of Ca2+ signaling to reduce excitability of RGS2-/- cells. J Biol Chem. 2004; 279:41642–41649. PMID: 15292238.20. Shin DM, Dehoff M, Luo X, Kang SH, Tu J, Nayak SK, Ross EM, Worley PF, Muallem S. Homer 2 tunes G protein-coupled receptors stimulus intensity by regulating RGS proteins and PLCbeta GAP activities. J Cell Biol. 2003; 162:293–303. PMID: 12860966.21. Ruiz de Azua I, Scarselli M, Rosemond E, Gautam D, Jou W, Gavrilova O, Ebert PJ, Levitt P, Wess J. RGS4 is a negative regulator of insulin release from pancreatic beta-cells in vitro and in vivo. Proc Natl Acad Sci USA. 2010; 107:7999–8004. PMID: 20385802.22. Zeng W, Lee MG, Yan M, Diaz J, Benjamin I, Marino CR, Kopito R, Freedman S, Cotton C, Muallem S, Thomas P. Immuno and functional characterization of CFTR in submandibular and pancreatic acinar and duct cells. Am J Physiol. 1997; 273:C442–C455. PMID: 9277342.
Article23. Bernfeld P. Enzymes of starch degradation and synthesis. Adv Enzymol Relat Subj Biochem. 1951; 12:379–428. PMID: 14885023.
Article24. Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000; 69:795–827. PMID: 10966476.
Article25. Hollinger S, Hepler JR. Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol Rev. 2002; 54:527–559. PMID: 12223533.
Article26. Giacomello M, Drago I, Pizzo P, Pozzan T. Mitochondrial Ca2+ as a key regulator of cell life and death. Cell Death Differ. 2007; 14:1267–1274. PMID: 17431419.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Ca2+-dependent Synaptotagmin Isoforms in Mouse and Rat Parotid Acinar Cells
- Regulation of the expression and function of TRPCs and Orai1 by Homer2 in mouse pancreatic acinar cells
- Modulation of InsP3 receptor properties by phosphorylation: targeting of PKA to InsP3 receptors shapes oscillatory calcium signals in pancreatic acinar cells
- Homer2 regulates amylase secretion via physiological calcium oscillations in mouse parotid gland acinar cells
- Role of Homeostatic Changes in Salivary Gland Acinar Cells in Primary Sjöögren's Syndrome: A Review