Yonsei Med J.
2006 Feb;47(1):70-77. 10.3349/ymj.2006.47.1.70.
Expression of Ca2+-dependent Synaptotagmin Isoforms in Mouse and Rat Parotid Acinar Cells
- Affiliations
-
- 1Department of Oral Biology, Brain Korea 21 Project of Medical Science, Yonsei University College of Dentistry, Seoul, Korea.
- 2Department of Pedodontics, Yonsei University College of Dentistry, Seoul, Korea. dmshin@yumc.yonsei.ac.kr
- KMID: 1715875
- DOI: http://doi.org/10.3349/ymj.2006.47.1.70
Abstract
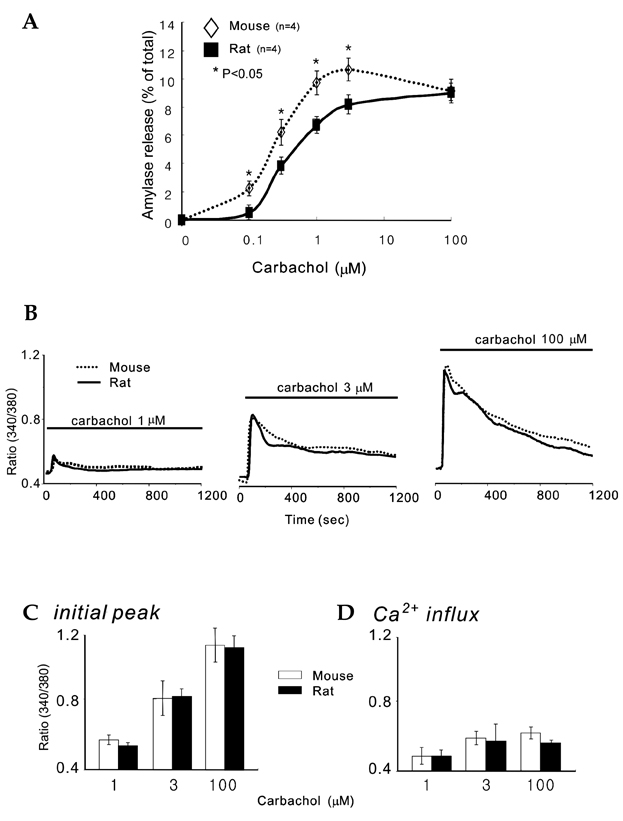
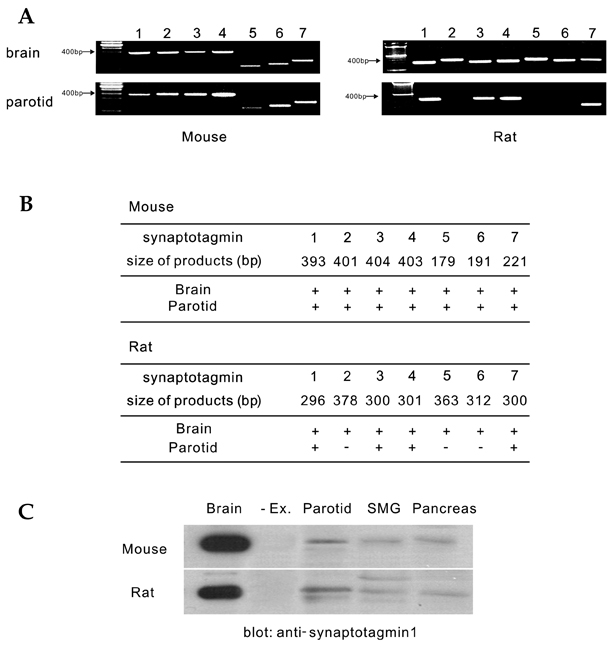
- Synaptotagmin is a Ca2+ sensing protein, which triggers a fusion of synaptic vesicles in neuronal transmission. Little is known regarding the expression of Ca2+ - dependent synaptotagmin isoforms and their contribution to the release of secretory vesicles in mouse and rat parotid acinar cells. We investigated a type of Ca2+ - dependent synaptotagmin and Ca2+ signaling in both rat and mouse parotid acinar cells using RT-PCR, microfluorometry, and amylase assay. Mouse parotid acinar cells exhibited much more sensitive amylase release in response to muscarinic stimulation than did rat parotid acinar cells. However, transient [Ca2+]i increases and Ca2+ influx in response to muscarinic stimulation in both cells were identical, suggesting that the expression or activity of the Ca2+ sensing proteins is different. Seven Ca2+ - dependent synaptotagmins, from 1 to 7, were expressed in the mouse parotid acinar cells. However, in the rat parotid acinar cells, only synaptotagmins 1, 3, 4 and 7 were expressed. These results indicate that the expression of Ca2+ - dependent synaptotagmins may contribute to the release of secretory vesicles in parotid acinar cells.
MeSH Terms
Figure
Reference
-
1. Quissell DO. Dobrosielski-Vergona K, editor. Stimulus-exocytosis coupling mechanism in salivary gland cells. Biology of the Salivary Glands. 1993. Florida: CRC Press;181–200.2. Takuma T, Ichida T. Catalytic subunit of protein kinase A induces amylase release from streptolysin O-permeabilized parotid acini. J Biol Chem. 1994. 269:22124–22128.3. Turner RJ, Sugiya H. Understanding salivary fluid and protein secretion. Oral Dis. 2002. 8:3–11.4. Takuma T, Ichida T. Does cyclic AMP mobilize calcium for amylase secretion from rat parotid cells? Biochim Biophys Acta. 1986. 887:113–117.5. Butcher FR, Putney JW. Regulation of parotid gland function by cyclic nucleotides and calcium. Adv Cyclic Nucleotide Res. 1980. 13:215–249.6. Sugiya H, Furuyama S. The activation of Ca2+ mobilizing receptors in salivary gland. Biomed Res. 1989. 10:111–121.7. Hay JC, Scheller RH. SNAREs and NSF in targeted membrane fusion. Curr Opin Cell Biol. 1997. 9:505–512.8. Jahn R, Sudhof TC. Membrane fusion and exocytosis. Annu Rev Biochem. 1999. 68:863–911.9. Weber T. SNAREpins: minimal machinery for membrane fusion. Cell. 1998. 92:759–772.10. Mcnew JA. Compartmental specificity of cellular mem brane fusion encoded in SNARE protein. Nature. 2000. 407:153–159.11. Geppert M, Sudhof TC. RAB3 and synaptotagmin: the yin and yang of synaptic membrane fusion. Annu Rev Neurosci. 1998. 21:75–95.12. Fukuda M. Molecular cloning and characterization of human, rat and mouse synaptotagmin XV. Biochem Biophys Res Commun. 2003. 306:64–71.13. Butz S, Fernandez CR, Schmitz F, Jahn R, Sudohf TC. The subcellular localization of atypical synaptotagmin III and VI. Synaptotagmin III enriched in synapses and synaptic plasma membrane but not in synaptic vesicles. J Biol Chem. 1999. 274:18290–18296.14. Brown H, Meister B, Deeney J, Corkey BE, Yang SN, Larsson O, et al. Synaptotagmin III isoform is compartmentalized in pancreatic-cells and has a functional role in exocytosis. Diabetes. 2000. 49:383–391.15. Gao Z, Reavey CJ, Young RA, Jegier P, Wolf BA. Synaptotagmin III/VII isoforms mediate Ca2+ induced insulin secretion in pancreatic islet β cells. J Biol Chem. 2000. 275:36079–36085.16. Mitzuda M. Localization and functional role of synaptotagmin in insulin secretory vesicles in pancreatic-cells. Diabetes. 1997. 46:2002–2006.17. Takuma T, Arakawa T, Tajima Y. Interaction of SNARE proteins in rat parotid acinar cells. Arch Oral Biol. 2000. 45:369–375.18. Imai A, Nashida T, Shimomura H. mRNA expression of membrane-fusion-related proteins in rat parotid gland. Arch Oral Biol. 2001. 46:955–962.19. Zeng W, Lee MG, Yan M, Diaz J, Benzamin I, Marino CR, et al. Immuno and functional characterization of CFTR in submandibular and pancreatic acinar and duct cells. Am J Physiol. 1997. 273:C442–C455.20. Xu X, Diaz J, Zhao H, Muallem S. Chacterization, localization and axial distribution of calcium signaling receptors in the rat submandibular salivary glands ducts. J Physiol (Lond). 1996. 491:647–662.21. Hong JH, Lee SI, Kim KE, Yong TS, Seo JT, Sohn MH, et al. German cockroach extract activates protease-activated receptor 2 in human airway epithelial cells. J Allergy Clin Immunol. 2004. 113:315–319.22. Bernfeld P. Enzymes of starch degradation and synthesis. Adv Enzymol Relat Subj Biochem. 1951. 12:379–428.23. Li C, Ullrich B, Zhang JZ, Anderson RG, Brose N, Sudhof TC, et al. Calcium dependent and independent activities of neural and non-neural synaptotagmin. Nature. 1995. 375:594–599.24. Saegusa C, Fukuda M, Mikoshiba K. Synaptotagmin V is targeted to dense-core vesicles that undergo calcium dependent exocytosis in PC12 cells. J Biol Chem. 2002. 277:24499–24505.25. Zhang Q, Fukuda M, Bocktaele EV, Pascual O, Haydon PG. Synaptotagmin IV regulates glial glutamate release. Proc Natl Acad Sci USA. 2004. 101:9441–9446.26. Levious O, Feinstein N, Linial M. Expression and localization of synaptotagmin 1 in rat parotid gland. Eur J Cell Biol. 1997. 73:81–92.27. Sudhof TC. Synaptotagmins: Why so many? J Biol Chem. 2002. 277:7629–7632.28. Chapman ER. Synaptotagmin a calcium sensor that triggers exocytosis? Nat Rev Mol Cell Biol. 2002. 3:498–508.29. Zhao XS, Shin DM, Liu LH, Shull GE, Muallem S. Plasticity and adaptation of Ca2+ signaling and Ca2+-dependeny exocytosis in SERCA2+/- mice. EMBO J. 2001. 20:2680–2689.30. Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003. 4:517–529.31. Kiselyov K, Shin DM, Muallem S. Signaling specificity in GPCR-dependent calcium signaling. Cell Signal. 2003. 15:243–253.32. Shin DM, Luo X, Wilkie TM, Miller LJ, Peck AB, Humphreys-Beher MG. Polarized expression of G protein-coupled receptors and an all-or-none discharge of Ca2+ pools at initiation sites of [Ca2+]i waves in polarized exocrine cells. J Biol Chem. 2001. 276:44146–44156.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Regulators of G-Protein Signaling 4 in Ca2+ Signaling in Mouse Pancreatic Acinar Cells
- Alteration of Expression of Ca(2+) Signaling Proteins and Adaptation of Ca(2+) Signaling in SERCA2(+/-) Mouse Parotid Acini
- Differential Expression and Cellular Localization of Na+-K+-ATPase Isoforms in Rat Salivary Gland
- Initiation Site of Ca2+ Entry Evoked by Endoplasmic Reticulum Ca2+ Depletion in Mouse Parotid and Pancreatic Acinar Cells
- Activation of PKCdelta by tyrosine phosphorylation in rat parotid acinar cells