Cancer Res Treat.
2024 Oct;56(4):1136-1145. 10.4143/crt.2023.1324.
Varlitinib and Paclitaxel for EGFR/HER2 Co-expressing Advanced Gastric Cancer: A Multicenter Phase Ib/II Study (K-MASTER-13)
- Affiliations
-
- 1Divison of Hematology/Oncology, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Division of Medical Oncology, Department of Internal Medicine, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea
- 3Division of Medical Oncology/Hematology, Department of Internal Medicine, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- 4Division of Medical Oncology, Department of Internal Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 5Division of Hematology-Oncology, Department of Internal Medicine, Hallym University Medical Center, Hallym University College of Medicine, Anyang, Korea
- 6Division of Hematology-Oncology, Department of Internal Medicine, Chonnam National University Hwasun Hospital, Chonnam National University Medical School, Hwasun, Korea
- 7Department of Pathology, Yonsei University College of Medicine, Seoul, Korea
- 8Songdang Institute for Cancer Research, Yonsei University College of Medicine, Seoul, Korea
- 9Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2560248
- DOI: http://doi.org/10.4143/crt.2023.1324
Abstract
- Purpose
Varlitinib is a pan-human epidermal growth factor receptor (HER) inhibitor targeting epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2), and HER4. We present a phase Ib/II study of a combination of varlitinib and weekly paclitaxel as a second-line treatment for patients with EGFR/HER2 co-expressing advanced gastric cancer (AGC).
Materials and Methods
Patients whose tumors with EGFR and HER2 overexpression by immunohistochemistry (≥ 1+) were enrolled. Varlitinib and paclitaxel were investigated every 4 weeks. After determining the recommended phase II dose (RP2D) in phase Ib, a phase II study was conducted to evaluate the antitumor activity.
Results
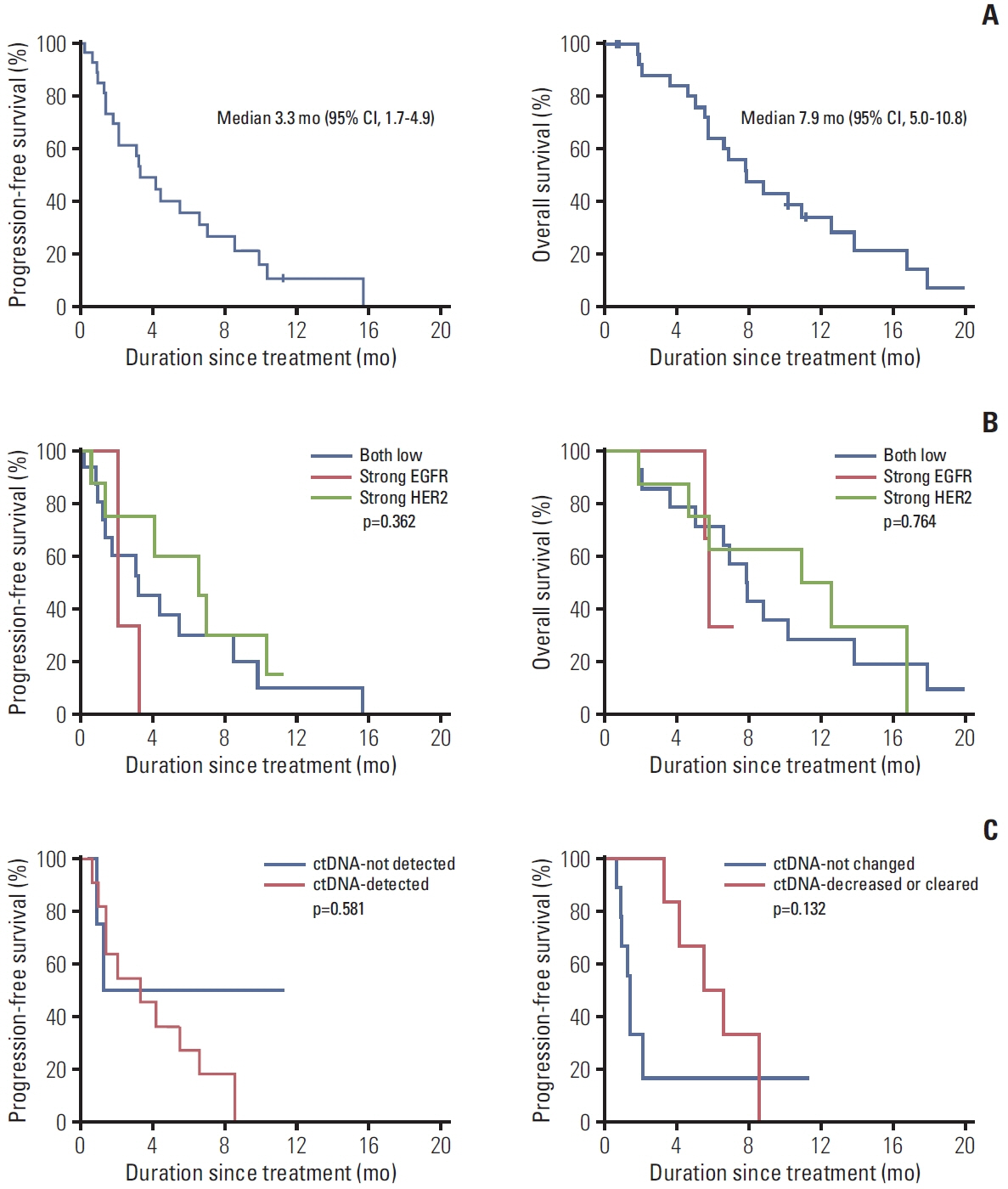
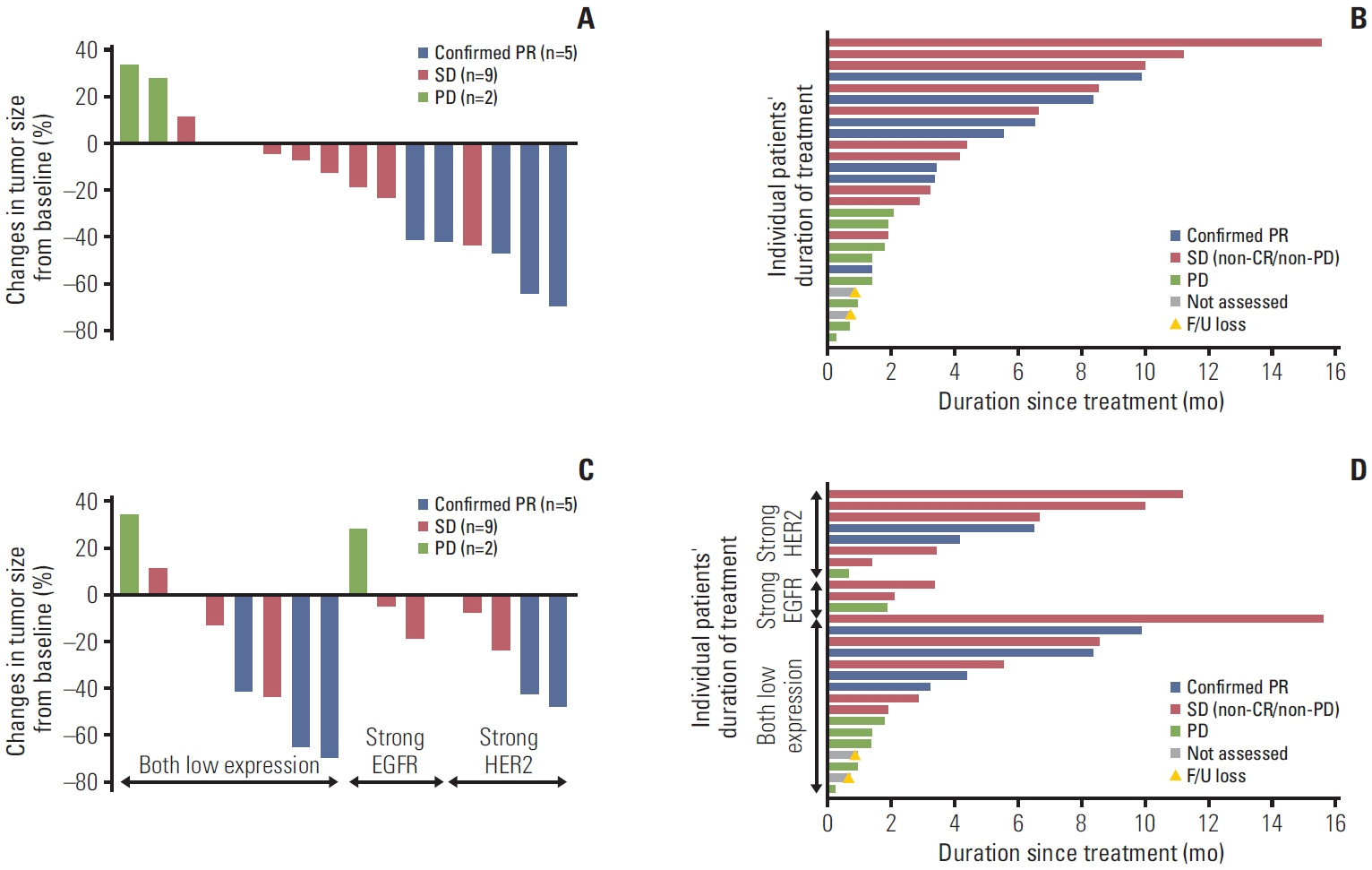
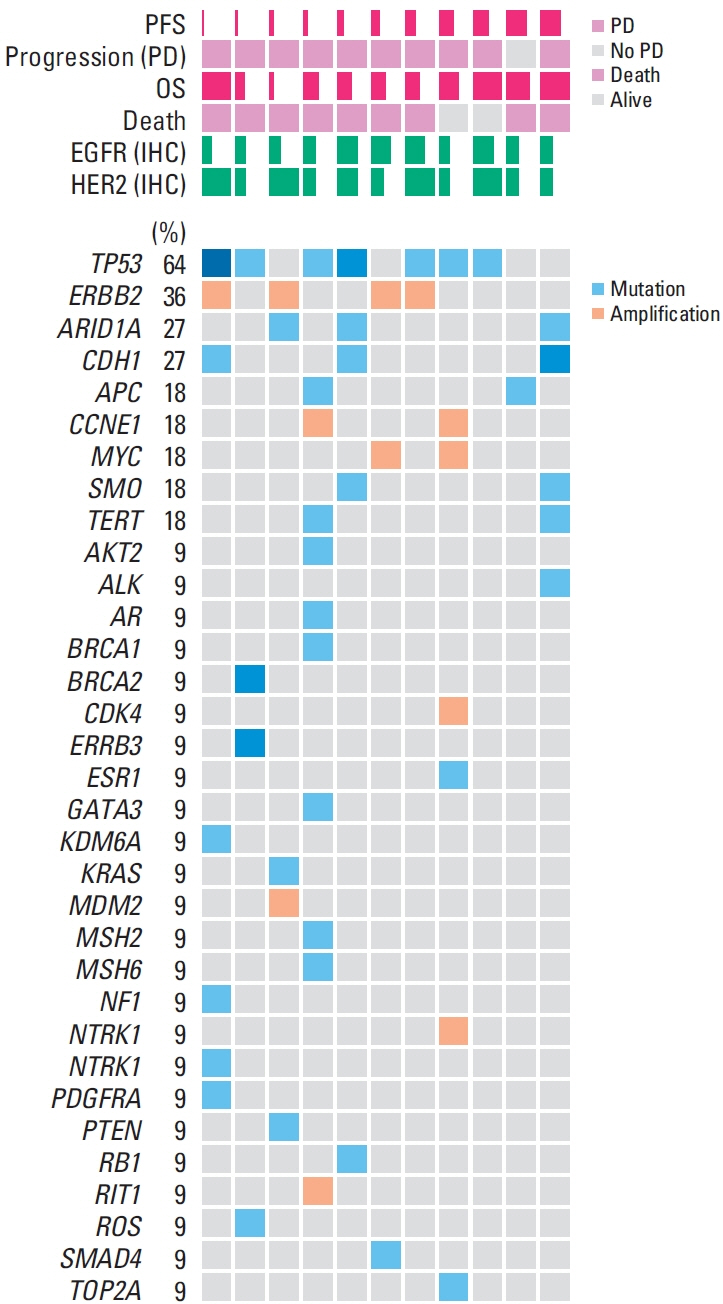
RP2D was treated with a combination of varlitinib (300 mg twice daily) and paclitaxel. Among 27 patients treated with RP2D, the median progression-free survival and overall survival (OS) were 3.3 months (95% confidence interval [CI], 1.7 to 4.9) and 7.9 months (95% CI, 5.0 to 10.8), respectively, with a median follow-up of 15.7 months. Among 16 patients with measurable disease, the objective response rate (ORR) and disease control rate were 31% and 88%, respectively. Patients with strong HER2 expression (n=8) had a higher ORR and longer OS, whereas those with strong EGFR expression (n=3) had poorer outcomes. The most common adverse events (AEs) of any grade were neutropenia (52%), diarrhea (27%), aspartate aminotransferase/alanine transaminase elevation (22%), and nausea (19%). No treatment-related deaths or unexpected AEs resulting from treatment cessation were observed in patients with RP2D.
Conclusion
A combination of varlitinib and paclitaxel displayed manageable toxicity and modest antitumor activity in patients with EGFR/HER2 co-expressing AGC who progressed after first-line chemotherapy.
Figure
Reference
-
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–49.
Article2. Kim TH, Kim IH, Kang SJ, Choi M, Kim BH, Eom BW, et al. Korean Practice Guidelines for Gastric Cancer 2022: an evidence-based, multidisciplinary approach. J Gastric Cancer. 2023; 23:3–106.
Article3. Koo DH, Ryu MH, Lee MY, Chae H, Kim EJ, Moon MS, et al. Trends in chemotherapy patterns and survival of patients with advanced gastric cancer over a 16-year period: impact of anti-HER2-targeted agent in the real-world setting. Cancer Res Treat. 2021; 53:436–44.
Article4. Hudis CA. Trastuzumab: mechanism of action and use in clinical practice. N Engl J Med. 2007; 357:39–51.
Article5. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2- positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010; 376:687–97.6. Rha SY, Chung HC. Breakthroughs in the systemic treatment of HER2-positive advanced/metastatic gastric cancer: from singlet chemotherapy to triple combination. J Gastric Cancer. 2023; 23:224–49.
Article7. Kim CG, Jung M, Kim HS, Lee CK, Jeung HC, Koo DH, et al. Trastuzumab combined with ramucirumab and paclitaxel in patients with previously treated human epidermal growth factor receptor 2-positive advanced gastric or gastroesophageal junction cancer. J Clin Oncol. 2023; 41:4394–405.
Article8. Henjes F, Bender C, von der Heyde S, Braun L, Mannsperger HA, Schmidt C, et al. Strong EGFR signaling in cell line models of ERBB2-amplified breast cancer attenuates response towards ERBB2-targeting drugs. Oncogenesis. 2012; 1:e16.
Article9. Satoh T, Xu RH, Chung HC, Sun GP, Doi T, Xu JM, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the secondline treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol. 2014; 32:2039–49.10. Iqbal S, Goldman B, Fenoglio-Preiser CM, Lenz HJ, Zhang W, Danenberg KD, et al. Southwest Oncology Group study S0413: a phase II trial of lapatinib (GW572016) as first-line therapy in patients with advanced or metastatic gastric cancer. Ann Oncol. 2011; 22:2610–5.
Article11. Oh DY, Lee KW, Cho JY, Kang WK, Im SA, Kim JW, et al. Phase II trial of dacomitinib in patients with HER2-positive gastric cancer. Gastric Cancer. 2016; 19:1095–103.
Article12. Martin N, Isambert N, Gomez-Roca C, Goeldner RG, Zanetta S, Sadrolhefazi B, et al. Phase I trial of afatinib and 3-weekly trastuzumab with optimal anti-diarrheal management in patients with HER2-positive metastatic cancer. Cancer Chemother Pharmacol. 2018; 82:979–86.
Article13. Dokduang H, Jamnongkarn W, Promraksa B, Suksawat M, Padthaisong S, Thanee M, et al. In vitro and in vivo antitumor effects of pan-HER inhibitor varlitinib on cholangiocarcinoma cell lines. Drug Des Devel Ther. 2020; 14:2319–34.14. Sierke SL, Cheng K, Kim HH, Koland JG. Biochemical characterization of the protein tyrosine kinase homology domain of the ErbB3 (HER3) receptor protein. Biochem J. 1997; 322(Pt 3):757–63.
Article15. Ooi AG, Lindmark BE, McHale M, Hung HT, Ong R. Varlitinib demonstrates potent antitumor efficacy in patient-derived gastric cancer xenograft models. Cancer Res. 2016; 76(14 Suppl):4719.
Article16. Liu CY, Chu PY, Huang CT, Chen JL, Yang HP, Wang WL, et al. Varlitinib downregulates HER/ERK signaling and induces apoptosis in triple negative breast cancer cells. Cancers (Basel). 2019; 11:105.
Article17. Kim J, Im S, Lee K, Kim JW, Lee K, Han S, et al. 664P: phase Iia study to evaluate the biological activity of Aslan001 in Her1/2 co-expressing or Her-2 amplified advanced gastric cancer. Ann Oncol. 2014; 25(Suppl 4):iv226.18. Lee MX, Wong ALA, Ow S, Sundar R, Tan DSP, Soo RA, et al. Phase Ib dose-finding study of varlitinib combined with weekly paclitaxel with or without carboplatin +/- trastuzumab in advanced solid tumors. Target Oncol. 2022; 17:141–51.
Article19. Javle MM, Oh DY, Ikeda M, Yong WP, Hsu K, Lindmark B, et al. Varlitinib plus capecitabine in second-line advanced biliary tract cancer: a randomized, phase II study (TreeTopp). ESMO Open. 2022; 7:100314.
Article20. Jeong SH, Kyung D, Yuk HD, Jeong CW, Lee W, Yoon JK, et al. Practical utility of liquid biopsies for evaluating genomic alterations in castration-resistant prostate cancer. Cancers (Basel). 2023; 15:2847.
Article21. Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014; 15:1224–35.
Article22. Nakamura Y, Kawazoe A, Lordick F, Janjigian YY, Shitara K. Biomarker-targeted therapies for advanced-stage gastric and gastro-oesophageal junction cancers: an emerging paradigm. Nat Rev Clin Oncol. 2021; 18:473–87.
Article23. Lee KS, Seo J, Lee CK, Shin S, Choi Z, Min S, et al. Analytical and clinical validation of cell-free circulating tumor DNA assay for the estimation of tumor mutational burden. Clin Chem. 2022; 68:1519–28.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Phase II Study of Paclitaxel and Cisplatin as Salvage Therapy for Patients with Advanced or Metastatic Gastric Cancer
- GASTric Cancer HER2 Re-Assessment Study 2 (GASTHER2): HER2 Re-assessment for Initially HER2-Negative Advanced Gastric Cancer Patients after Progression on First-Line Treatment
- Diagnosis and Treatment of HER2-Positive Breast Cancer
- A Phase II Trial of Paclitaxel, 5-fluorouracil (5-FU) and Cisplatin in Patients with Metastatic or Recurrent Gastric Cancer
- Multicenter Retrospective Analysis of Intraperitoneal Paclitaxel and Systemic Chemotherapy for Advanced Gastric Cancer with Peritoneal Metastasis