J Gastric Cancer.
2020 Mar;20(1):50-59. 10.5230/jgc.2020.20.e6.
Multicenter Retrospective Analysis of Intraperitoneal Paclitaxel and Systemic Chemotherapy for Advanced Gastric Cancer with Peritoneal Metastasis
- Affiliations
-
- 1Department of Surgery, Dankook University Hospital, Cheonan, Korea. ysjee@dkuh.co.kr
- 2Department of Surgery, Incheon St. Mary's Hospital, Incheon, Korea.
- 3Department of Surgery, Korea University College of Medicine, Seoul, Korea.
- 4Department of Surgery, Kyung Hee University Hospital at Gangdong, Seoul, Korea.
- 5Department of Surgery, Chung-Ang University College of Medicine, Seoul, Korea.
- KMID: 2471925
- DOI: http://doi.org/10.5230/jgc.2020.20.e6
Abstract
- PURPOSE
The objective of the present retrospective analysis was to describe the experience of intraperitoneal (IP) paclitaxel and systemic chemotherapy in patients with peritoneal metastasis (PM) of advanced gastric cancer (AGC) in a multicenter setting in Korea.
MATERIALS AND METHODS
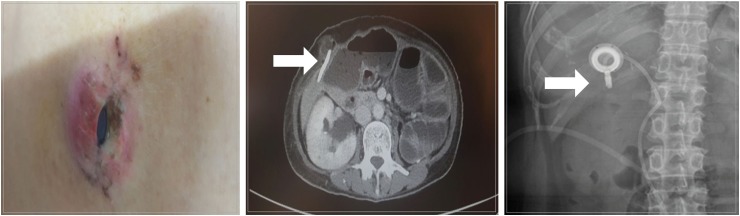
The medical records of patients with AGC, who were diagnosed with PM between January 2015 and December 2018, were reviewed. IP catheter was placed in the pouch of Douglas and was used for the administration of IP paclitaxel chemotherapy.
RESULTS
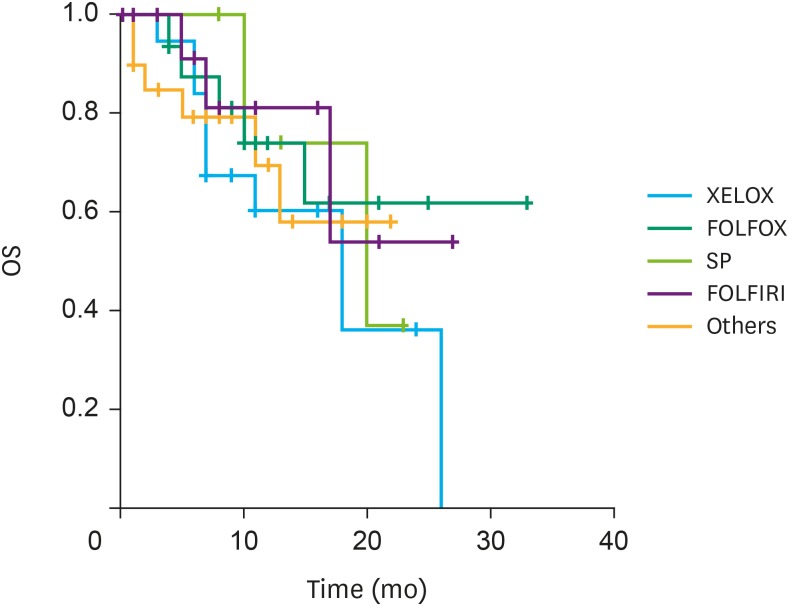
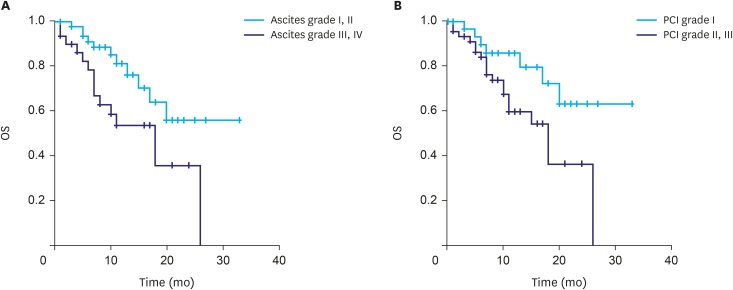
We reviewed the clinical outcomes of IP paclitaxel and systemic chemotherapy administration in 82 patients at six institutions in Korea. Mean number of IP chemotherapy cycles was 6.6. The mean peritoneal cancer index (PCI) was 21.9. Postoperative complications related to IP catheter and port were observed in 15 patients. The overall median survival was 20.0 months. A significant difference was observed in the survival rate according to the ascites grade (grade I and II, 24.1 months; grade III and IV, 15.3 months; P=0.014) and PCI grade (grade I, 25.6 months; grade II and III, 16.3 months; P=0.023).
CONCLUSIONS
The feasibility of IP paclitaxel and systemic chemotherapy administration was demonstrated in this experience-based retrospective analysis suggesting that the procedure is beneficial in patients with PM of AGC.
MeSH Terms
Figure
Cited by 1 articles
-
Treatment options for advanced gastric cancer with peritoneal metastasis: experience from a single institution in Korea
Dong-Wook Kim, Sang Il Youn, Ye Seob Jee
Ann Surg Treat Res. 2021;100(4):209-217. doi: 10.4174/astr.2021.100.4.209.
Reference
-
1. Lee JH, Son SY, Lee CM, Ahn SH, Park DJ, Kim HH. Factors predicting peritoneal recurrence in advanced gastric cancer: implication for adjuvant intraperitoneal chemotherapy. Gastric Cancer. 2014; 17:529–536. PMID: 24101155.
Article2. Guideline Committee of the Korean Gastric Cancer Association (KGCA), Development Working Group & Review Panel. Korean Practice Guideline for Gastric Cancer 2018: an evidence-based, multi-disciplinary approach. J Gastric Cancer. 2019; 19:1–48. PMID: 30944757.3. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017; 20:1–19.4. Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008; 9:215–221. PMID: 18282805.
Article5. Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009; 20:666–673. PMID: 19153121.
Article6. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010; 376:687–697. PMID: 20728210.
Article7. Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014; 15:1224–1235. PMID: 25240821.
Article8. Kitayama J, Ishigami H, Yamaguchi H, Sakuma Y, Horie H, Hosoya Y, et al. Treatment of patients with peritoneal metastases from gastric cancer. Ann Gastroenterol Surg. 2018; 2:116–123. PMID: 29863151.
Article9. Ishigami H, Kitayama J, Kaisaki S, Hidemura A, Kato M, Otani K, et al. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol. 2010; 21:67–70. PMID: 19605503.
Article10. Imano M, Yasuda A, Itoh T, Satou T, Peng YF, Kato H, et al. Phase II study of single intraperitoneal chemotherapy followed by systemic chemotherapy for gastric cancer with peritoneal metastasis. J Gastrointest Surg. 2012; 16:2190–2196. PMID: 23099736.
Article11. Yamaguchi H, Kitayama J, Ishigami H, Emoto S, Yamashita H, Watanabe T. A phase 2 trial of intravenous and intraperitoneal paclitaxel combined with S-1 for treatment of gastric cancer with macroscopic peritoneal metastasis. Cancer. 2013; 119:3354–3358. PMID: 23798046.
Article12. Ishigami H, Fujiwara Y, Fukushima R, Nashimoto A, Yabusaki H, Imano M, et al. Phase III trial comparing intraperitoneal and intravenous paclitaxel plus S-1 versus cisplatin plus S-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC trial. J Clin Oncol. 2018; 36:1922–1929. PMID: 29746229.
Article13. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–216. PMID: 10655437.14. Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996; 82:359–374. PMID: 8849962.
Article15. Yonemura Y, Sako S, Wakama S, Ishibashi H, Mizumoto A, Takao N, et al. History of peritoneal surface malignancy treatment in Japan. Indian J Surg Oncol. 2019; 10:3–11.
Article16. Kodera Y. Surgery with curative intent for stage IV gastric cancer: is it a reality of illusion? Ann Gastroenterol Surg. 2018; 2:339–347. PMID: 30238074.
Article17. Dedrick RL, Myers CE, Bungay PM, DeVita VT Jr. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep. 1978; 62:1–11. PMID: 626987.18. Jacquet P, Sugarbaker PH. Peritoneal-plasma barrier. Cancer Treat Res. 1996; 82:53–63. PMID: 8849943.
Article19. Kobayashi D, Kodera Y. Intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis. Gastric Cancer. 2017; 20:111–121. PMID: 27803990.
Article20. Cho H, Ryu MH, Kim KP, Ryoo BY, Park SR, Kim BS, et al. Phase I/II study of a combination of capecitabine, cisplatin, and intraperitoneal docetaxel (XP ID) in advanced gastric cancer patients with peritoneal metastasis. Gastric Cancer. 2017; 20:970–977. PMID: 28303362.
Article21. Fushida S, Kinoshita J, Yagi Y, Funaki H, Kinami S, Ninomiya I, et al. Dual anti-cancer effects of weekly intraperitoneal docetaxel in treatment of advanced gastric cancer patients with peritoneal carcinomatosis: a feasibility and pharmacokinetic study. Oncol Rep. 2008; 19:1305–1310. PMID: 18425392.
Article22. Imano M, Peng YF, Itoh T, Nishikawa M, Satou T, Yasuda A, et al. A preliminary study of single intraperitoneal administration of paclitaxel followed by sequential systemic chemotherapy with S-1 plus paclitaxel for advanced gastric cancer with peritoneal metastasis. Anticancer Res. 2012; 32:4071–4075. PMID: 22993363.23. Emoto S, Ishigami H, Hidemura A, Yamaguchi H, Yamashita H, Kitayama J, et al. Complications and management of an implanted intraperitoneal access port system for intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis. Jpn J Clin Oncol. 2012; 42:1013–1019. PMID: 22872745.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic Value of Early Postoperative Intraperitoneal Chemotherapy in Resectable Advanced Gastric Cancer
- Prognostic Factors in Advanced Gastric Cancer with Peritoneal Carcinomatosis
- Intraperitoneal Paclitaxel Combined with S-1 Plus Oxaliplatin for Advanced Gastric Cancer with Peritoneal Metastasis: a Phase I Study
- Treatment options for advanced gastric cancer with peritoneal metastasis: experience from a single institution in Korea
- Combined Cytoreductive Surgery and Early Postoperative Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis of Gastric Cancer