Diabetes Metab J.
2024 May;48(3):405-417. 10.4093/dmj.2023.0196.
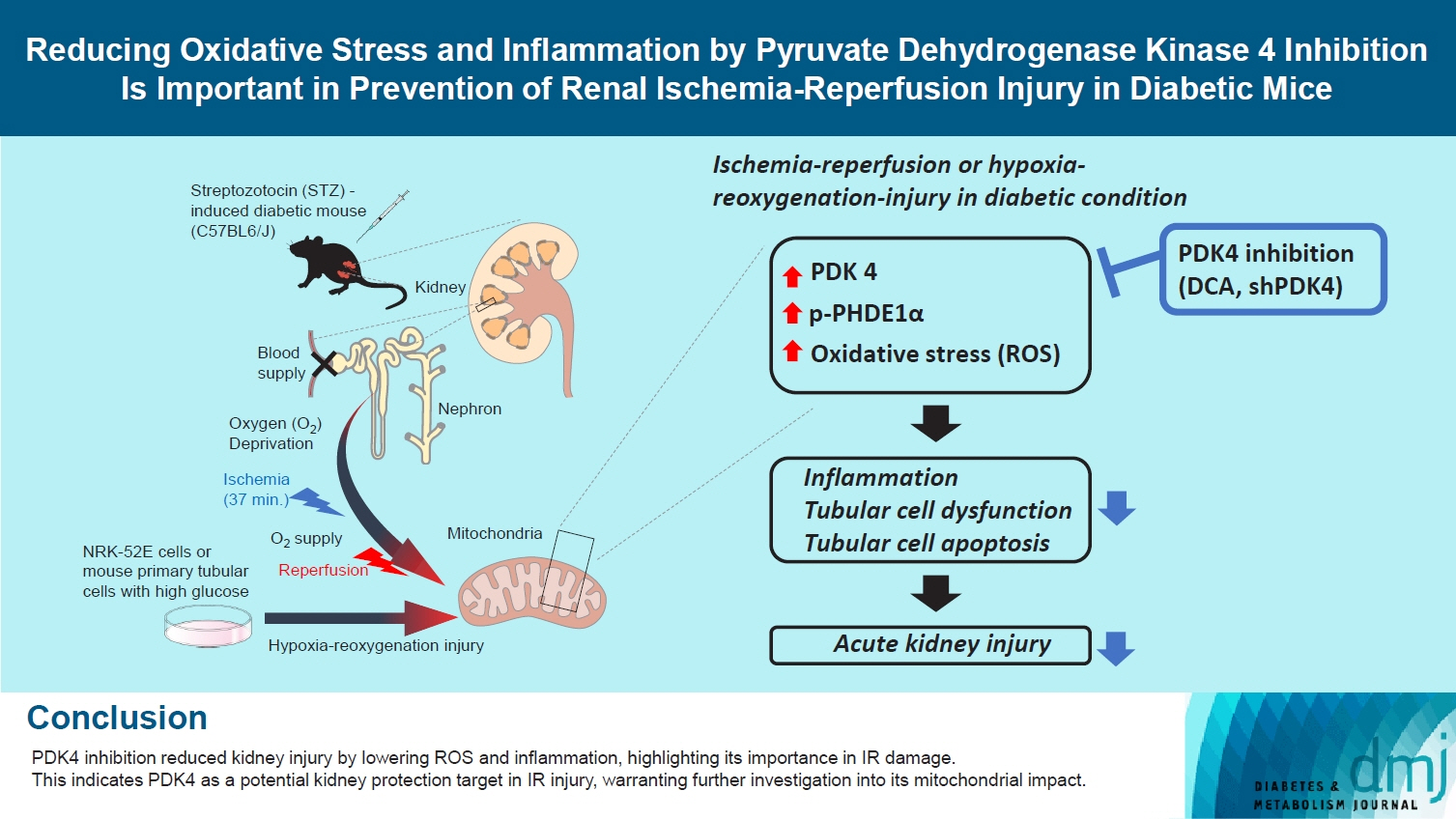
Reducing Oxidative Stress and Inflammation by Pyruvate Dehydrogenase Kinase 4 Inhibition Is Important in Prevention of Renal Ischemia-Reperfusion Injury in Diabetic Mice
- Affiliations
-
- 1Department of Internal Medicine, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Yangsan, Korea
- 2Department of Biomedical Science, Graduate School, Kyungpook National University, Daegu, Korea
- 3Department of Internal Medicine, Kyungpook National University Chilgok Hospital, School of Medicine, Kyungpook National University, Daegu, Korea
- 4Research Institute of Aging and Metabolism, School of Medicine, Kyungpook National University, Daegu, Korea
- 5Leading-edge Research Center for Drug Discovery and Development for Diabetes and Metabolic Disease, Kyungpook National University Hospital, Daegu, Korea
- 6Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 7Department of Internal Medicine, Kyungpook National University Hospital, School of Medicine, Kyungpook National University, Daegu, Korea
- KMID: 2555772
- DOI: http://doi.org/10.4093/dmj.2023.0196
Abstract
- Background
Reactive oxygen species (ROS) and inflammation are reported to have a fundamental role in the pathogenesis of ischemia-reperfusion (IR) injury, a leading cause of acute kidney injury. The present study investigated the role of pyruvate dehydrogenase kinase 4 (PDK4) in ROS production and inflammation following IR injury.
Methods
We used a streptozotocin-induced diabetic C57BL6/J mouse model, which was subjected to IR by clamping both renal pedicles. Cellular apoptosis and inflammatory markers were evaluated in NRK-52E cells and mouse primary tubular cells after hypoxia and reoxygenation using a hypoxia work station.
Results
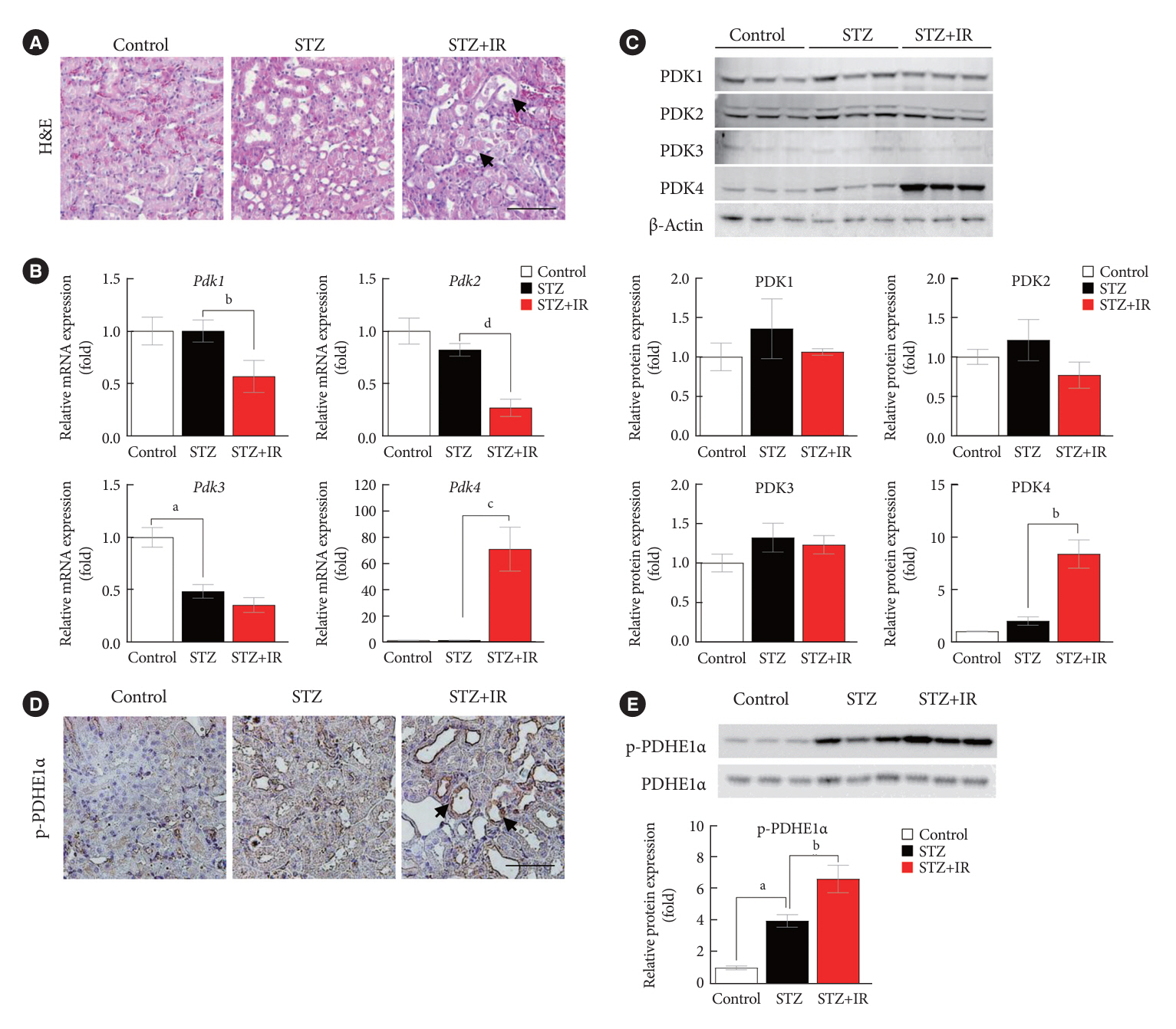
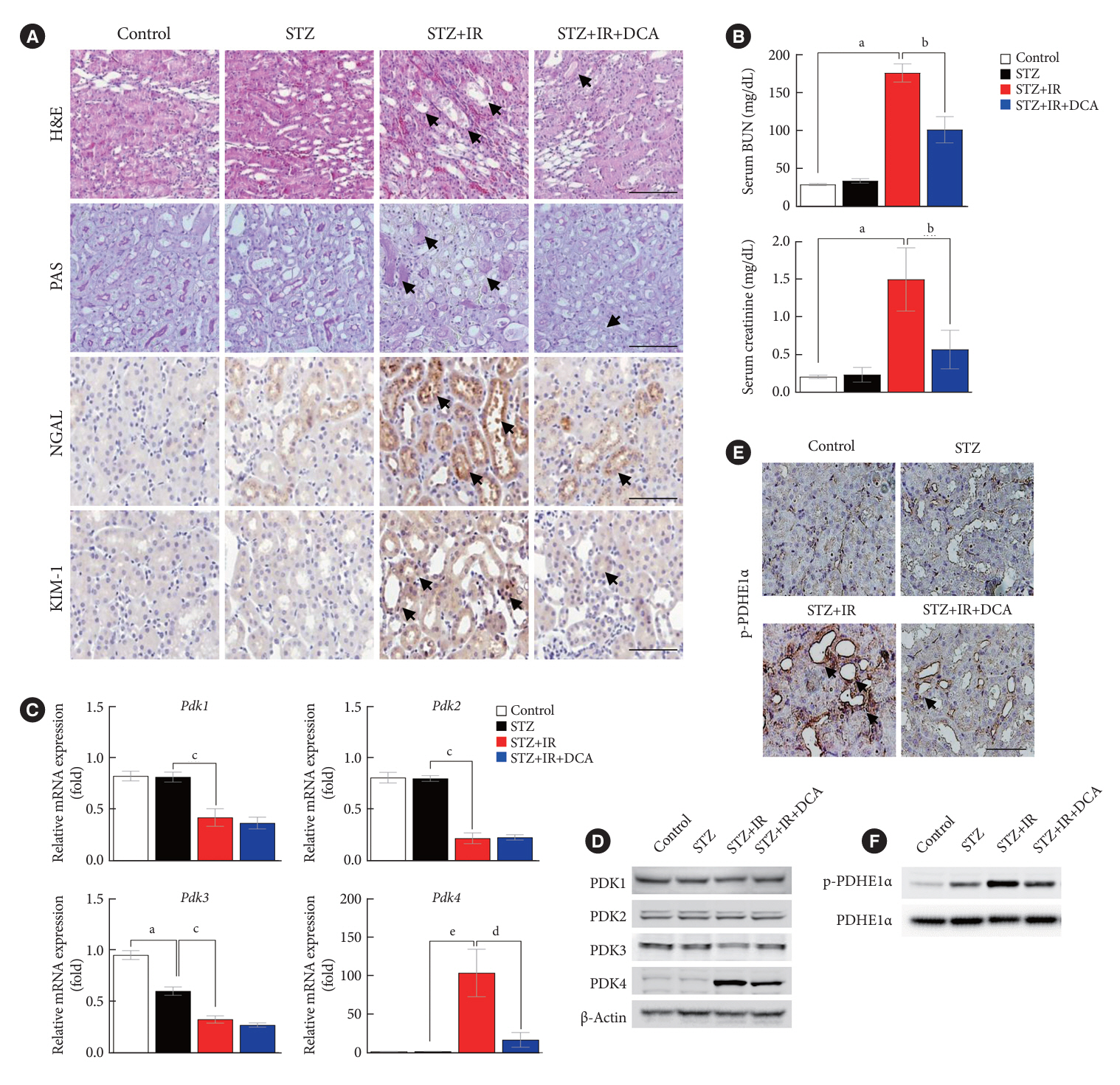
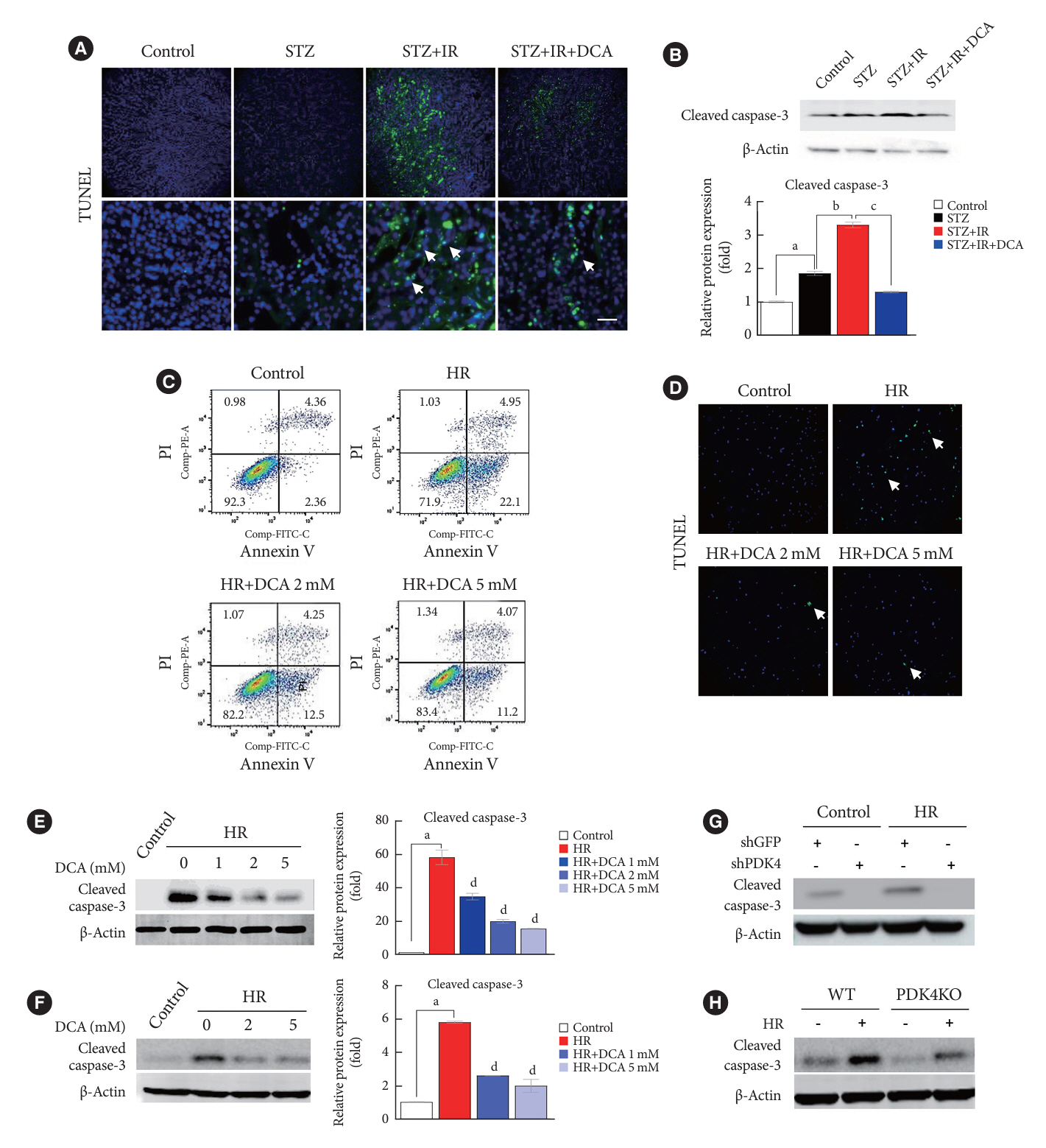
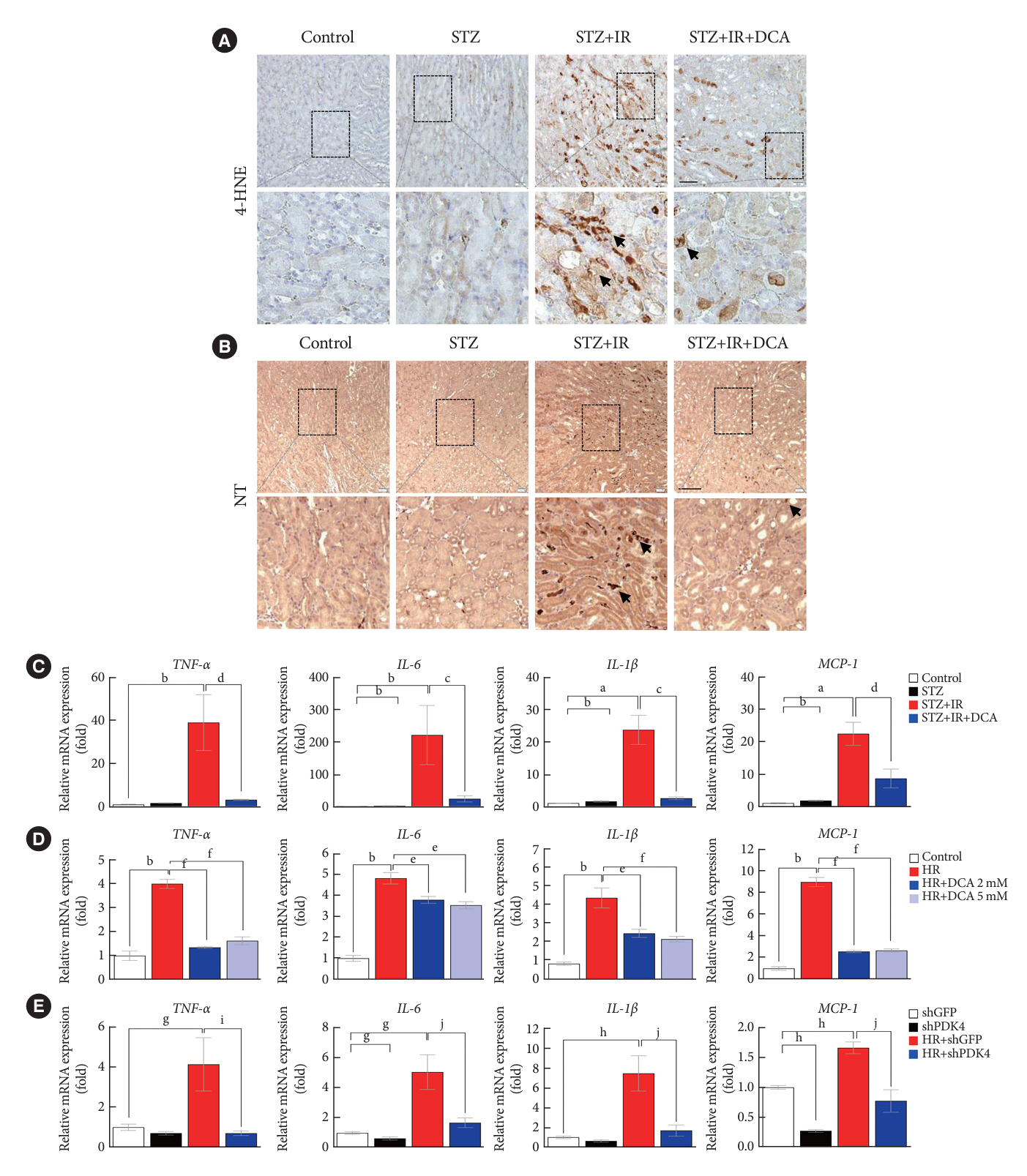
Following IR injury in diabetic mice, the expression of PDK4, rather than the other PDK isoforms, was induced with a marked increase in pyruvate dehydrogenase E1α (PDHE1α) phosphorylation. This was accompanied by a pronounced ROS activation, as well as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), interleukin-1β (IL-1β), and monocyte chemoattractant protein-1 (MCP-1) production. Notably, sodium dichloroacetate (DCA) attenuated renal IR injury-induced apoptosis which can be attributed to reducing PDK4 expression and PDHE1α phosphorylation levels. DCA or shPdk4 treatment reduced oxidative stress and decreased TNF-α, IL-6, IL-1β, and MCP-1 production after IR or hypoxia-reoxygenation injury.
Conclusion
PDK4 inhibition alleviated renal injury with decreased ROS production and inflammation, supporting a critical role for PDK4 in IR mediated damage. This result indicates another potential target for reno-protection during IR injury; accordingly, the role of PDK4 inhibition needs to be comprehensively elucidated in terms of mitochondrial function during renal IR injury.
Keyword
Figure
Cited by 1 articles
-
Exploring Renal Pyruvate Metabolism as a Therapeutic Avenue for Diabetic Kidney Injury
Jaemin Lee
Diabetes Metab J. 2024;48(3):385-386. doi: 10.4093/dmj.2024.0210.
Reference
-
1. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Inter Suppl. 2012; 2:1–138.2. Hoste EA, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006; 10:R73.3. Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006; 34:1913–7.
Article4. Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012; 380:756–66.
Article5. Bienholz A, Wilde B, Kribben A. From the nephrologist’s point of view: diversity of causes and clinical features of acute kidney injury. Clin Kidney J. 2015; 8:405–14.
Article6. Eltzschig HK, Eckle T. Ischemia and reperfusion: from mechanism to translation. Nat Med. 2011; 17:1391–401.
Article7. Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013; 123:92–100.
Article8. Cavaille-Coll M, Bala S, Velidedeoglu E, Hernandez A, Archdeacon P, Gonzalez G, et al. Summary of FDA workshop on ischemia reperfusion injury in kidney transplantation. Am J Transplant. 2013; 13:1134–48.
Article9. Lefer DJ, Bolli R. Development of an NIH consortium for preclinical AssESsment of CARdioprotective therapies (CAESAR): a paradigm shift in studies of infarct size limitation. J Cardiovasc Pharmacol Ther. 2011; 16:332–9.
Article10. Braunwald E, Kloner RA. Myocardial reperfusion: a doubleedged sword? J Clin Invest. 1985; 76:1713–9.
Article11. Hearse DJ, Humphrey SM, Bullock GR. The oxygen paradox and the calcium paradox: two facets of the same problem? J Mol Cell Cardiol. 1978; 10:641–68.
Article12. Raedschelders K, Ansley DM, Chen DD. The cellular and molecular origin of reactive oxygen species generation during myocardial ischemia and reperfusion. Pharmacol Ther. 2012; 133:230–55.
Article13. Soares RO, Losada DM, Jordani MC, Evora P, Castro-E-Silva O. Ischemia/reperfusion injury revisited: an overview of the latest pharmacological strategies. Int J Mol Sci. 2019; 20:5034.
Article14. Chouchani ET, Pell VR, James AM, Work LM, Saeb-Parsy K, Frezza C, et al. A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metab. 2016; 23:254–63.
Article15. Padanilam BJ. Cell death induced by acute renal injury: a perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol. 2003; 284:F608–27.
Article16. Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014; 515:431–5.
Article17. Rabb H, Griffin MD, McKay DB, Swaminathan S, Pickkers P, Rosner MH, et al. Inflammation in AKI: current understanding, key questions, and knowledge gaps. J Am Soc Nephrol. 2016; 27:371–9.18. Andrianova NV, Zorov DB, Plotnikov EY. Targeting inflammation and oxidative stress as a therapy for ischemic kidney injury. Biochemistry (Mosc). 2020; 85:1591–602.
Article19. Jeoung NH. Pyruvate dehydrogenase kinases: therapeutic targets for diabetes and cancers. Diabetes Metab J. 2015; 39:188–97.
Article20. Sugden MC, Holness MJ. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am J Physiol Endocrinol Metab. 2003; 284:E855–62.
Article21. Oh CJ, Ha CM, Choi YK, Park S, Choe MS, Jeoung NH, et al. Pyruvate dehydrogenase kinase 4 deficiency attenuates cisplatin-induced acute kidney injury. Kidney Int. 2017; 91:880–95.
Article22. Park BY, Jeon JH, Go Y, Ham HJ, Kim JE, Yoo EK, et al. PDK4 deficiency suppresses hepatic glucagon signaling by decreasing cAMP levels. Diabetes. 2018; 67:2054–68.
Article23. Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004; 66:480–5.
Article24. Okusa MD. The inflammatory cascade in acute ischemic renal failure. Nephron. 2002; 90:133–8.
Article25. Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006; 3:177–85.
Article26. Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006; 3:187–97.
Article27. Lu CW, Lin SC, Chen KF, Lai YY, Tsai SJ. Induction of pyruvate dehydrogenase kinase-3 by hypoxia-inducible factor-1 promotes metabolic switch and drug resistance. J Biol Chem. 2008; 283:28106–14.
Article28. Prigione A, Rohwer N, Hoffmann S, Mlody B, Drews K, Bukowiecki R, et al. HIF1α modulates cell fate reprogramming through early glycolytic shift and upregulation of PDK1-3 and PKM2. Stem Cells. 2014; 32:364–76.29. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009; 324:1029–33.
Article30. Wu P, Blair PV, Sato J, Jaskiewicz J, Popov KM, Harris RA. Starvation increases the amount of pyruvate dehydrogenase kinase in several mammalian tissues. Arch Biochem Biophys. 2000; 381:1–7.
Article31. Wu P, Sato J, Zhao Y, Jaskiewicz J, Popov KM, Harris RA. Starvation and diabetes increase the amount of pyruvate dehydrogenase kinase isoenzyme 4 in rat heart. Biochem J. 1998; 329(Pt 1):197–201.
Article32. Jeoung NH, Wu P, Joshi MA, Jaskiewicz J, Bock CB, Depaoli-Roach AA, et al. Role of pyruvate dehydrogenase kinase isoenzyme 4 (PDHK4) in glucose homoeostasis during starvation. Biochem J. 2006; 397:417–25.
Article33. Klyuyeva A, Tuganova A, Kedishvili N, Popov KM. Tissue-specific kinase expression and activity regulate flux through the pyruvate dehydrogenase complex. J Biol Chem. 2019; 294:838–51.
Article34. Sugden MC, Holness MJ. Mechanisms underlying regulation of the expression and activities of the mammalian pyruvate dehydrogenase kinases. Arch Physiol Biochem. 2006; 112:139–49.
Article35. Harris RA, Huang B, Wu P. Control of pyruvate dehydrogenase kinase gene expression. Adv Enzyme Regul. 2001; 41:269–88.
Article36. Go Y, Jeong JY, Jeoung NH, Jeon JH, Park BY, Kang HJ, et al. Inhibition of pyruvate dehydrogenase kinase 2 protects against hepatic steatosis through modulation of tricarboxylic acid cycle anaplerosis and ketogenesis. Diabetes. 2016; 65:2876–87.
Article37. Lee SJ, Jeong JY, Oh CJ, Park S, Kim JY, Kim HJ, et al. Pyruvate dehydrogenase kinase 4 promotes vascular calcification via SMAD1/5/8 phosphorylation. Sci Rep. 2015; 5:16577.
Article38. Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordonez JD, Go AS. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009; 4:891–8.
Article39. Thakar CV, Christianson A, Himmelfarb J, Leonard AC. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol. 2011; 6:2567–72.
Article40. Goor Y, Peer G, Iaina A, Blum M, Wollman Y, Chernihovsky T, et al. Nitric oxide in ischaemic acute renal failure of streptozotocin diabetic rats. Diabetologia. 1996; 39:1036–40.
Article41. Peng J, Li X, Zhang D, Chen JK, Su Y, Smith SB, et al. Hyperglycemia, p53, and mitochondrial pathway of apoptosis are involved in the susceptibility of diabetic models to ischemic acute kidney injury. Kidney Int. 2015; 87:137–50.
Article42. Kelly KJ, Burford JL, Dominguez JH. Postischemic inflammatory syndrome: a critical mechanism of progression in diabetic nephropathy. Am J Physiol Renal Physiol. 2009; 297:F923–31.
Article43. Gao G, Zhang B, Ramesh G, Betterly D, Tadagavadi RK, Wang W, et al. TNF-a mediates increased susceptibility to ischemic AKI in diabetes. Am J Physiol Renal Physiol. 2013; 304:F515–21.44. Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008; 214:199–210.
Article45. Lloyd CM, Minto AW, Dorf ME, Proudfoot A, Wells TN, Salant DJ, et al. RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J Exp Med. 1997; 185:1371–80.
Article46. Schwarz M, Wahl M, Resch K, Radeke HH. IFNgamma induces functional chemokine receptor expression in human mesangial cells. Clin Exp Immunol. 2002; 128:285–94.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ethyl Pyruvate Ameliorates Renal Ischemia- reperfusion Injury
- Hepatic ischemia-reperfusion injury with respect to oxidative stress and inflammatory response: a narrative review
- SP600125, a selective JNK inhibitor, aggravates hepatic ischemia-reperfusion injury
- Brivaracitam Ameliorates Increased Inflammation, Oxidative Stress, and Acetylcholinesterase Activity in Ischemic Mice
- Pyruvate Dehydrogenase Kinases: Therapeutic Targets for Diabetes and Cancers