Anesth Pain Med.
2023 Jul;18(3):275-283. 10.17085/apm.23021.
Chronic exposure to dexamethasone may not affect sugammadex reversal of rocuronium-induced neuromuscular blockade: an in vivo study on rats
- Affiliations
-
- 1Independent Scholar, Seoul, Korea
- 2Department of Anesthesiology and Pain Medicine, Inje University Seoul Paik Hospital, Seoul, Korea
- 3Department of Anesthesia and Pain Medicine, Gachon University Gil Medical Center, Incheon, Korea
- 4Department of Anesthesiology and Pain Medicine, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- 5Department of Anesthesiology and Pain Medicine, Dongguk University Ilsan Hospital, Goyang, Korea
- 6Department of Anesthesiology and Pain Medicine, Daejeon Eulji Medical Center, Eulji University School of Medicine, Daejeon, Korea
- KMID: 2547036
- DOI: http://doi.org/10.17085/apm.23021
Abstract
- Background
Chronic glucocorticoid exposure is associated with resistance to nondepolarizing neuromuscular blocking agents. Therefore, we hypothesized that sugammadex-induced recovery would occur more rapidly in subjects exposed to chronic dexamethasone compared to those who were not exposed. This study evaluated the sugammadex-induced recovery profile after neuromuscular blockade (NMB) in rats exposed to chronic dexamethasone.
Methods
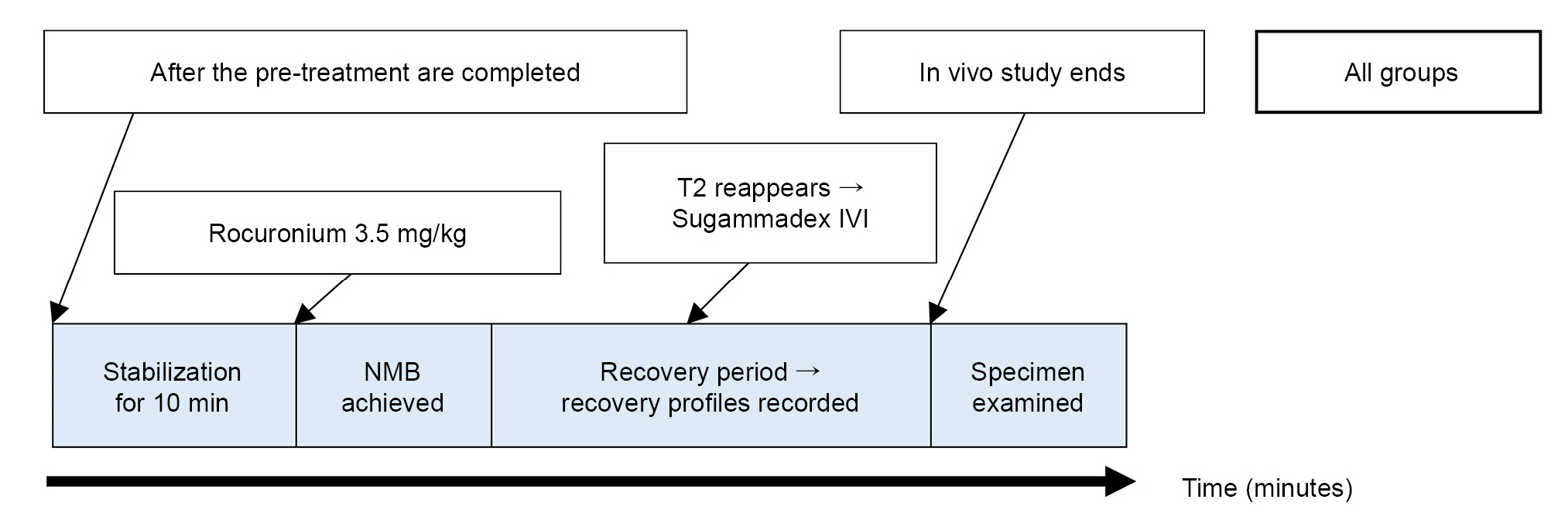
Sprague–Dawley rats were allocated to three groups (dexamethasone, control, and pair-fed group) for the in vivo study. The mice received daily intraperitoneal dexamethasone injections (500 μg/kg) or 0.9% saline for 15 days. To achieve complete NMB, 3.5 mg/kg rocuronium was administered on the sixteenth day. The recovery time to a train-of-four ratio ≥ 0.9 was measured to evaluate the complete recovery following the sugammadex injection.
Results
Among the groups, no significant differences were observed in the recovery time to a train-of-four ratio ≥ 0.9 following sugammadex administration (P = 0.531). The time to the second twitch of the train-of-four recovery following rocuronium administration indicated that the duration of NMB was significantly shorter in Group D than that in Groups C and P (P = 0.001).
Conclusions
Chronic exposure to dexamethasone did not shorten the recovery time of sugammadex-induced NMB reversal. However, the findings of this study indicated that no adjustments to sugammadex dosage or route of administration is required, even in patients undergoing long-term steroid treatment.
Keyword
Figure
Reference
-
1. Wallimann P, Marti T, Fürer A, Diederich F. Steroids in molecular recognition. Chem Rev. 1997; 97:1567–608.
Article2. Suy K, Morias K, Cammu G, Hans P, Van Duijnhoven WG, Heeringa M, et al. Effective reversal of moderate rocuronium- or vecuronium-induced neuromuscular block with sugammadex, a selective relaxant binding agent. Anesthesiology. 2007; 106:283–8.
Article3. Goldstein MF, Fallon JJ, Harning R. Chronic glucocorticoid therapy-induced osteoporosis in patients with obstructive lung disease. Chest. 1999; 116:1733–49.
Article4. Soltész S, Mencke T, Mey C, Röhrig S, Diefenbach C, Molter GP. Influence of a continuous prednisolone medication on the time course of neuromuscular block of atracurium in patients with chronic inflammatory bowel disease. Br J Anaesth. 2008; 100:798–802.
Article5. Soltész S, Mencke T, Stunz M, Diefenbach C, Ziegeler S, Molter GP. Attenuation of a rocuronium-induced neuromuscular block in patients receiving prednisolone. Acta Anaesthesiol Scand. 2009; 53:443–8.
Article6. Chen D, Yang MR, Huang LN, Qiu YW, Li ST. Different magnitude of resistance to non-depolarizing muscle relaxants in dexamethasone-treated rat diaphragm associated with altered acetylcholine receptor expression. Genet Mol Res. 2014; 13:5892–900.
Article7. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010; 8:e1000412.
Article8. Rezonja K, Lorenzon P, Mars T. Opposing effects of dexamethasone, agrin and sugammadex on functional innervation and constitutive secretion of IL-6 in in vitro innervated primary human muscle cells. Neurosci Lett. 2013; 549:186–90.
Article9. Itoh H, Shibata K, Matsumoto T, Nitta S, Nishi M, Kobayashi T, et al. Effects of neuromuscular-blocking drugs in rats in vivo: direct measurements in the diaphragm and tibialis anterior muscle. Acta Anaesthesiol Scand. 2004; 48:903–8.
Article10. Haerter F, Simons JC, Foerster U, Moreno Duarte I, Diaz-Gil D, Ganapati S, et al. Comparative effectiveness of calabadion and sugammadex to reverse non-depolarizing neuromuscular-blocking agents. Anesthesiology. 2015; 123:1337–49.
Article11. Weinger MB, Partridge BL, Henry AF. Dexmedetomidine does not modify the neuromuscular blocking action of vecuronium in the anaesthetized rat. Br J Anaesth. 1995; 74:455–7.12. Chen D, Yang MR, Huang LN, Qiu YW, Li ST. Dexamethasone‑induced hyposensitivity to rocuronium in rat diaphragm associated with muscle‑fiber transformation. Mol Med Rep. 2014; 9:527–34.
Article13. Yamamoto D, Maki T, Herningtyas EH, Ikeshita N, Shibahara H, Sugiyama Y, et al. Branched-chain amino acids protect against dexamethasone-induced soleus muscle atrophy in rats. Muscle Nerve. 2010; 41:819–27.
Article14. Arenillas M, Gomez de Segura IA. Anaesthetic effects of alfaxalone administered intraperitoneally alone or combined with dexmedetomidine and fentanyl in the rat. Lab Anim. 2018; 52:588–98.
Article15. Eikermann M, Zaremba S, Malhotra A, Jordan AS, Rosow C, Chamberlin NL. Neostigmine but not sugammadex impairs upper airway dilator muscle activity and breathing. Br J Anaesth. 2008; 101:344–9.
Article16. Dardevet D, Sornet C, Grizard J. Glucocorticoid-induced insulin resistance of protein synthesis is independent of the rapamycin-sensitive pathways in rat skeletal muscle. J Endocrinol. 1999; 162:77–85.
Article17. Rieu I, Sornet C, Grizard J, Dardevet D. Glucocorticoid excess induces a prolonged leucine resistance on muscle protein synthesis in old rats. Exp Gerontol. 2004; 39:1315–21.
Article18. Hoffmann U, Grosse-Sundrup M, Eikermann-Haerter K, Zaremba S, Ayata C, Zhang B, et al. Calabadion: a new agent to reverse the effects of benzylisoquinoline and steroidal neuromuscular-blocking agents. Anesthesiology. 2013; 119:317–25.19. Samtani MN, Jusko WJ. Comparison of dexamethasone pharmacokinetics in female rats after intravenous and intramuscular administration. Biopharm Drug Dispos. 2005; 26:85–91.
Article20. Lee S, Yang HS, Sasakawa T, Khan MA, Khatri A, Kaneki M, et al. Immobilization with atrophy induces de novo expression of neuronal nicotinic α7 acetylcholine receptors in muscle contributing to neurotransmission. Anesthesiology. 2014; 120:76–85.
Article21. Osta WA, El-Osta MA, Pezhman EA, Raad RA, Ferguson K, Mckelvey GM, et al. Nicotinic acetylcholine receptor gene expression is altered in burn patients. Anesth Analg. 2010; 110:1355–9.
Article22. Gupta A, Gupta Y. Glucocorticoid-induced myopathy: pathophysiology, diagnosis, and treatment. Indian J Endocrinol Metab. 2013; 17:913–6.
Article23. Ma D, Zhang B, Hoffmann U, Sundrup MG, Eikermann M, Isaacs L. Acyclic cucurbit [n] uril‐type molecular containers bind neuromuscular blocking agents in vitro and reverse neuromuscular block in vivo. Angewandte Chemie. 2012; 124:11520–4.24. Woo T, Kim KS, Shim YH, Kim MK, Yoon SM, Lim YJ, et al. Sugammadex versus neostigmine reversal of moderate rocuronium-induced neuromuscular blockade in Korean patients. Korean J Anesthesiol. 2013; 65:501–7.
Article25. Fuchs-Buder T, Meistelman C, Raft J. Sugammadex: clinical development and practical use. Korean J Anesthesiol. 2013; 65:495–500.
Article26. Jones RK, Caldwell JE, Brull SJ, Soto RG. Reversal of profound rocuronium-induced blockade with sugammadex: a randomized comparison with neostigmine. Anesthesiology. 2008; 109:816–24.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of dexamethasone and hydrocortisone on rocuronium-induced neuromuscular blockade and reversal by sugammadex in phrenic nerve-hemidiaphragm rat model
- Clinical use of sugammadex
- Advantages and pitfalls of clinical application of sugammadex
- Sugammadex: clinical development and practical use
- In the hour of Sugammadex