Int J Stem Cells.
2022 Aug;15(3):311-323. 10.15283/ijsc21173.
Immunomodulatory Effect of Epidermal Growth Factor Secreted by Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells on Atopic Dermatitis
- Affiliations
-
- 1Stem Cell and Regenerative Bioengineering Institute, Global R&D Center, Kangstem Biotech Co., Ltd., Seoul, Korea
- 2Adult Stem Cell Research Center, Research Institute for Veterinary Science, College of Veterinary Medicine, Seoul National University, Seoul, Korea
- 3Research Institute for Veterinary Science, College of Veterinary Medicine, Seoul National University, Seoul, Korea
- KMID: 2532405
- DOI: http://doi.org/10.15283/ijsc21173
Abstract
- Background and Objectives
Human mesenchymal stem cells (MSCs) are emerging as a treatment for atopic dermatitis (AD), a chronic inflammatory skin disorder that affects a large number of people across the world. Treatment of AD using human umbilical cord blood-derived MSCs (hUCB-MSCs) has recently been studied. However, the mechanism underlying their effect needs to be studied continuously. Thus, the objective of this study was to investigate the immunomodulatory effect of epidermal growth factor (EGF) secreted by hUCB-MSCs on AD.
Methods and Results
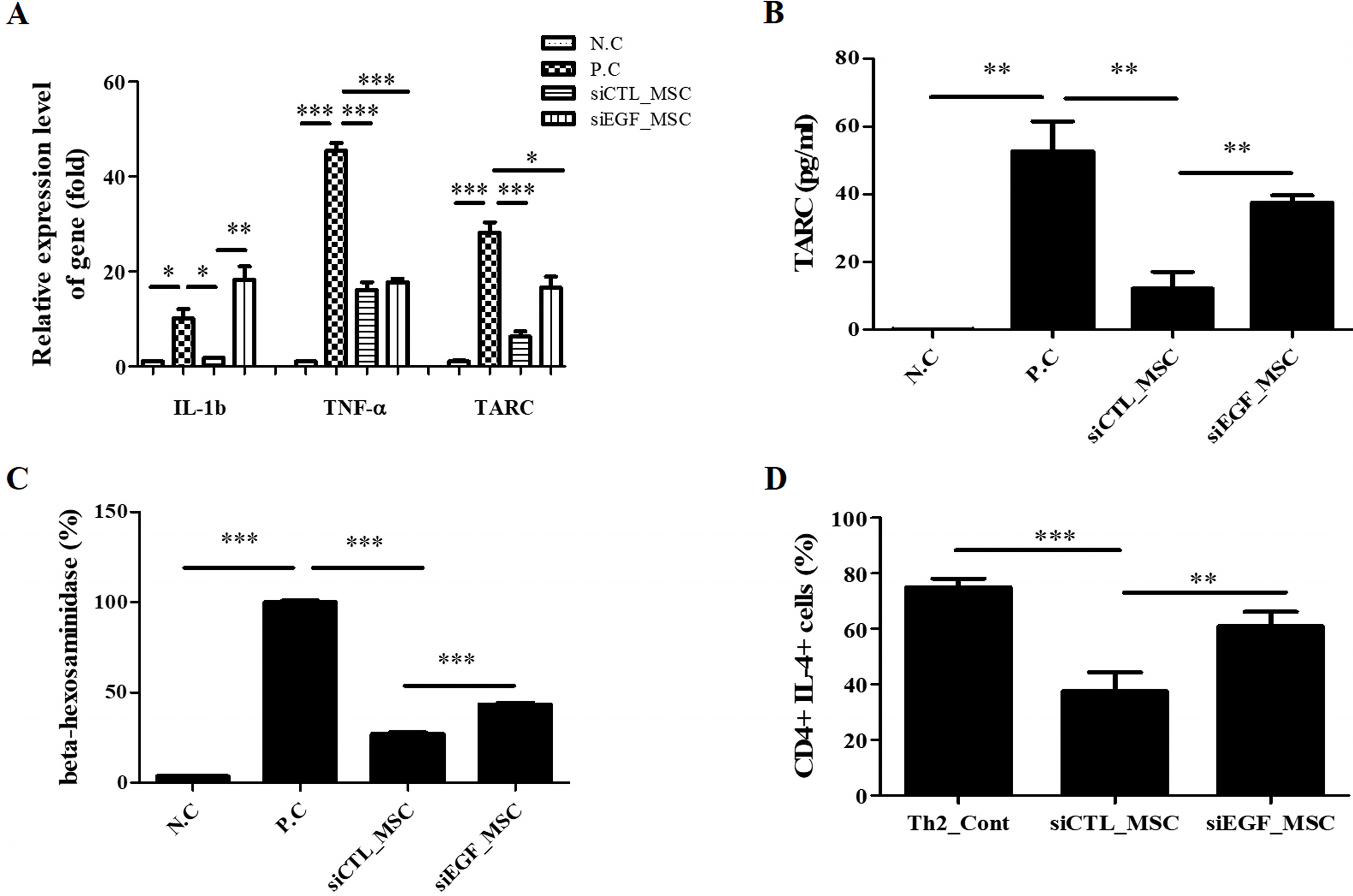
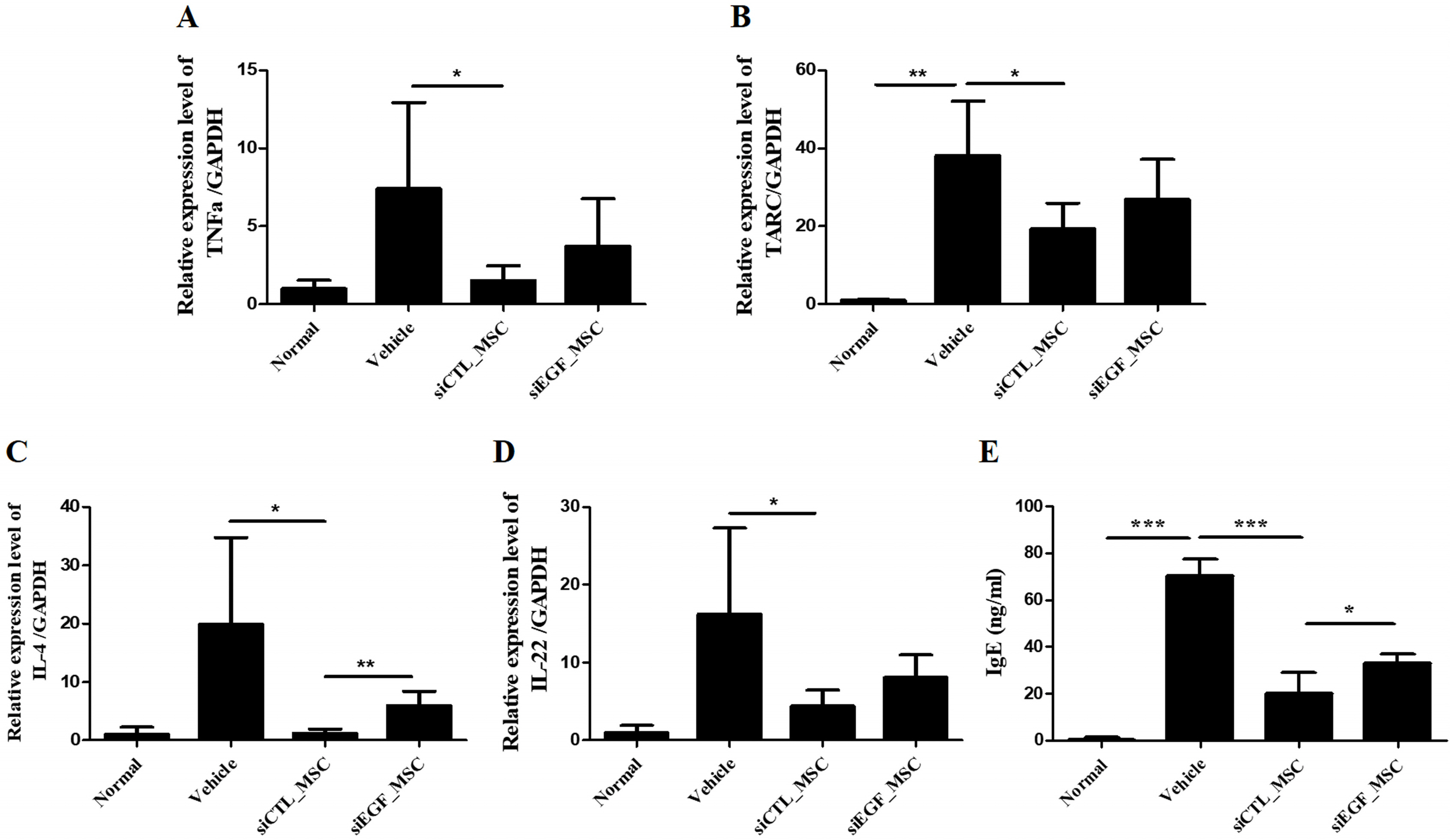
To explore the mechanism involved in the therapeutic effect of MSCs for AD, a secretome array was performed using culture medium of hUCB-MSCs. Among the list of genes common for epithelium development and skin diseases, we focused on the function of EGF. To elucidate the effect of EGF secreted by hUCB-MSCs, EGF was downregulated in hUCB-MSCs using EGF-targeting small interfering RNA. These cells were then co-cultured with keratinocytes, Th2 cells, and mast cells. Depletion of EGF disrupted immunomodulatory effects of hUCB-MSCs on these AD-related inflammatory cells. In a Dermatophagoides farinae-induced AD mouse model, subcutaneous injection of hUCB-MSCs ameliorated gross scoring, histopathologic damage, and mast cell infiltration. It also significantly reduced levels of inflammatory cytokines including interleukin (IL)-4, tumor necrosis factor (TNF)-α, thymus and activation-regulated chemokine (TARC), and IL-22, as well as IgE levels. These therapeutic effects were significantly attenuated at all evaluation points in mice injected with EGF-depleted hUCB-MSCs.
Conclusions
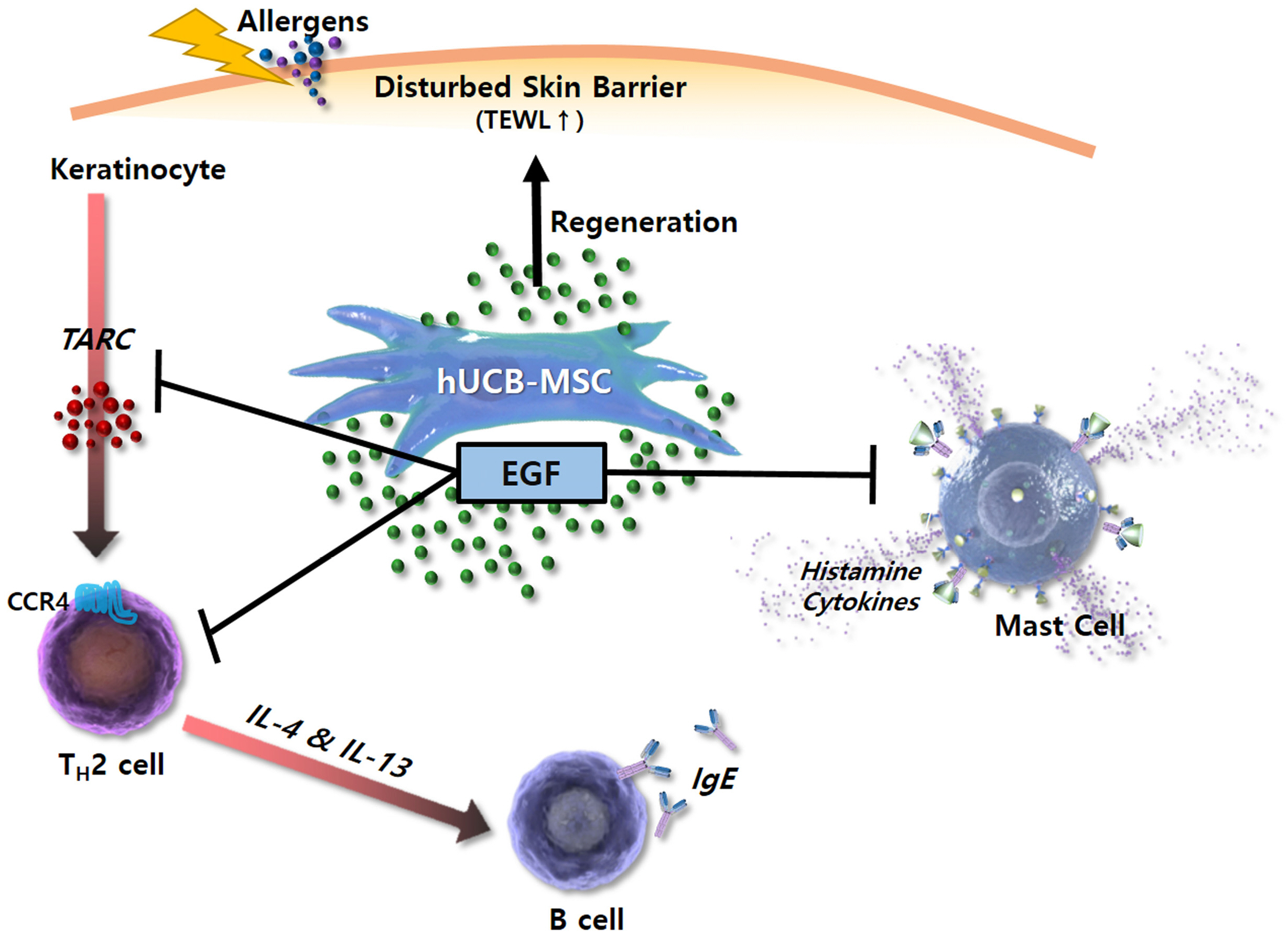
EGF secreted by hUCB-MSCs can improve AD by regulating inflammatory responses of keratinocytes, Th2 cells, and mast cells.
Keyword
Figure
Reference
-
References
1. Bantz SK, Zhu Z, Zheng T. 2014; The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. J Clin Cell Immunol. 5:202. DOI: 10.4172/2155-9899.1000202. PMID: 25419479. PMCID: PMC4240310.2. Daltro SRT, Meira CS, Santos IP, Ribeiro Dos Santos R, Soares MBP. 2020; Mesenchymal stem cells and atopic dermatitis: a review. Front Cell Dev Biol. 8:326. DOI: 10.3389/fcell.2020.00326. PMID: 32478072. PMCID: PMC7240073. PMID: fd999ad7be19495680b604708ba660bc. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85085522099&origin=inward.3. Biedermann T, Skabytska Y, Kaesler S, Volz T. 2015; Regulation of T cell immunity in atopic dermatitis by microbes: the Yin and Yang of cutaneous inflammation. Front Immunol. 6:353. DOI: 10.3389/fimmu.2015.00353. PMID: 26217343. PMCID: PMC4500098. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84935071781&origin=inward.4. Kakinuma T, Nakamura K, Wakugawa M, Mitsui H, Tada Y, Saeki H, Torii H, Asahina A, Onai N, Matsushima K, Tamaki K. 2001; Thymus and activation-regulated chemokine in atopic dermatitis: serum thymus and activation-regulated chemokine level is closely related with disease activity. J Allergy Clin Immunol. 107:535–541. DOI: 10.1067/mai.2001.113237. PMID: 11240957. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035098042&origin=inward.5. Vestergaard C, Bang K, Gesser B, Yoneyama H, Matsushima K, Larsen CG. 2000; A Th2 chemokine, TARC, produced by keratinocytes may recruit CLA+CCR4+ lymphocytes into lesional atopic dermatitis skin. J Invest Dermatol. 115:640–646. DOI: 10.1046/j.1523-1747.2000.00115.x. PMID: 10998136. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0033623179&origin=inward.6. Ritto D, Tanasawet S, Singkhorn S, Klaypradit W, Hutamekalin P, Tipmanee V, Sukketsiri W. 2017; Astaxanthin induces migration in human skin keratinocytes via Rac1 activation and RhoA inhibition. Nutr Res Pract. 11:275–280. DOI: 10.4162/nrp.2017.11.4.275. PMID: 28765773. PMCID: PMC5537536. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85026796948&origin=inward.7. Sah SK, Agrahari G, Nguyen CT, Kim YS, Kang KS, Kim TY. 2018; Enhanced therapeutic effects of human mesenchymal stem cells transduced with superoxide dismutase 3 in a murine atopic dermatitis-like skin inflammation model. Allergy. 73:2364–2376. DOI: 10.1111/all.13594. PMID: 30144097. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85054856705&origin=inward.8. Shigemoto-Kuroda T, Oh JY, Kim DK, Jeong HJ, Park SY, Lee HJ, Park JW, Kim TW, An SY, Prockop DJ, Lee RH. 2017; MSC-derived extracellular vesicles attenuate immune responses in two autoimmune murine models: type 1 diabetes and uveoretinitis. Stem Cell Reports. 8:1214–1225. DOI: 10.1016/j.stemcr.2017.04.008. PMID: 28494937. PMCID: PMC5425726. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85019447215&origin=inward.9. Ocansey DKW, Qiu W, Wang J, Yan Y, Qian H, Zhang X, Xu W, Mao F. 2020; The achievements and challenges of mesenchymal stem cell-based therapy in inflammatory bowel disease and its associated colorectal cancer. Stem Cells Int. 2020:7819824. DOI: 10.1155/2020/7819824. PMID: 32256612. PMCID: PMC7104387. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85082812746&origin=inward.10. Weiss ARR, Dahlke MH. 2019; Immunomodulation by mesenchymal stem cells (MSCs): mechanisms of action of living, apoptotic, and dead MSCs. Front Immunol. 10:1191. DOI: 10.3389/fimmu.2019.01191. PMID: 31214172. PMCID: PMC6557979. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85068432174&origin=inward.11. Puccinelli TJ, Bertics PJ, Masters KS. 2010; Regulation of keratinocyte signaling and function via changes in epidermal growth factor presentation. Acta Biomater. 6:3415–3425. DOI: 10.1016/j.actbio.2010.04.006. PMID: 20398806. PMCID: PMC2910130. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77956724792&origin=inward.12. Pastore S, Mascia F, Mariani V, Girolomoni G. 2008; The epidermal growth factor receptor system in skin repair and inflammation. J Invest Dermatol. 128:1365–1374. DOI: 10.1038/sj.jid.5701184. PMID: 18049451. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=43749094998&origin=inward.13. Sääf A, Pivarcsi A, Winge MC, Wahlgren CF, Homey B, Nordenskjöld M, Tengvall-Linder M, Bradley M. 2012; Characterization of EGFR and ErbB2 expression in atopic dermatitis patients. Arch Dermatol Res. 304:773–780. DOI: 10.1007/s00403-012-1242-4. PMID: 22552355. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84871246274&origin=inward.14. Erkasap S, Erkasap N, Aral E, Koken T, Kahraman A, Aydin Y, Yilmaz S, Ates E. 2005; Mast cell stabilizator and antioxidant effects of epidermal growth factor (EGF) on gastric mucosal injury induced by ethanol in rats. Chin J Physiol. 48:1–6. Erratum in: Chin J Physiol 2005;48:114. PMID: 15973961. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=15944411749&origin=inward.15. Jost M, Kari C, Rodeck U. 2000; The EGF receptor - an essential regulator of multiple epidermal functions. Eur J Dermatol. 10:505–510. PMID: 11056418. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0033762498&origin=inward.16. Jacob M, Bin Khalaf D, Alhissi S, Arnout R, Alsaud B, Al-Mousa H, Lopata AL, Alazami AM, Dasouki M, Abdel Rahman AM. 2019; Quantitative profiling of cytokines and chemokines in DOCK8-deficient and atopic dermatitis patients. Allergy. 74:370–379. DOI: 10.1111/all.13610. PMID: 30252138. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85054926979&origin=inward.17. Zhang Z, Xiao C, Gibson AM, Bass SA, Khurana Hershey GK. 2014; EGFR signaling blunts allergen-induced IL-6 production and Th17 responses in the skin and attenuates development and relapse of atopic dermatitis. J Immunol. 192:859–866. DOI: 10.4049/jimmunol.1301062. PMID: 24337738. PMCID: PMC3946981. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84893414859&origin=inward.18. Yu G. 2018; Using meshes for MeSH term enrichment and semantic analyses. Bioinformatics. 34:3766–3767. DOI: 10.1093/bioinformatics/bty410. PMID: 29790928. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85055600978&origin=inward.19. Saheli M, Bayat M, Ganji R, Hendudari F, Kheirjou R, Pakzad M, Najar B, Piryaei A. 2020; Human mesenchymal stem cells-conditioned medium improves diabetic wound healing mainly through modulating fibroblast behaviors. Arch Dermatol Res. 312:325–336. DOI: 10.1007/s00403-019-02016-6. PMID: 31786709. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85075688484&origin=inward.20. Sääf AM, Tengvall-Linder M, Chang HY, Adler AS, Wahlgren CF, Scheynius A, Nordenskjöld M, Bradley M. 2008; Global expression profiling in atopic eczema reveals reciprocal expression of inflammatory and lipid genes. PLoS One. 3:e4017. DOI: 10.1371/journal.pone.0004017. PMID: 19107207. PMCID: PMC2603322. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=58149177184&origin=inward.21. Kim YJ, Choi MJ, Bak DH, Lee BC, Ko EJ, Ahn GR, Ahn SW, Kim MJ, Na J, Kim BJ. 2018; Topical administration of EGF suppresses immune response and protects skin barrier in DNCB-induced atopic dermatitis in NC/Nga mice. Sci Rep. 8:11895. DOI: 10.1038/s41598-018-30404-x. PMID: 30093649. PMCID: PMC6085286. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85051465759&origin=inward.22. Troyer KL, Luetteke NC, Saxon ML, Qiu TH, Xian CJ, Lee DC. 2001; Growth retardation, duodenal lesions, and aberrant ileum architecture in triple null mice lacking EGF, amphiregulin, and TGF-alpha. Gastroenterology. 121:68–78. DOI: 10.1053/gast.2001.25478. PMID: 11438495. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0034950574&origin=inward.23. Mao Y, Ma J, Xia Y, Xie X. 2020; The overexpression of epidermal growth factor (EGF) in HaCaT cells promotes the proliferation, migration, invasion and transdifferentiation to epidermal stem cell immunophenotyping of adipose-derived stem cells (ADSCs). Int J Stem Cells. 13:93–103. DOI: 10.15283/ijsc18146. PMID: 32114740. PMCID: PMC7119215. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85084152368&origin=inward.24. Xia Y, You XE, Chen H, Yan YJ, He YC, Ding SZ. 2017; Epidermal growth factor promotes mesenchymal stem cell-mediated wound healing and hair follicle regeneration. Int J Clin Exp Pathol. 10:7390–7400. PMID: 31966581. PMCID: PMC6965296.25. Lin L, Nonoyama S, Oshiba A, Kabasawa Y, Mizutani S. 2003; TARC and MDC are produced by CD40 activated human B cells and are elevated in the sera of infantile atopic dermatitis patients. J Med Dent Sci. 50:27–33. PMID: 12715916. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0038209791&origin=inward.26. Vestergaard C, Deleuran M, Gesser B, Larsen CG. 2004; Thymus- and activation-regulated chemokine (TARC/CCL17) induces a Th2-dominated inflammatory reaction on intradermal injection in mice. Exp Dermatol. 13:265–271. DOI: 10.1111/j.0906-6705.2004.00149.x. PMID: 15086343. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=2442437020&origin=inward.27. Choi SY, Lee YJ, Kim JM, Kang HJ, Cho SH, Chang SE. 2018; Epidermal growth factor relieves inflammatory signals in Staphylococcus aureus-treated human epidermal keratinocytes and atopic dermatitis-like skin lesions in Nc/Nga mice. Biomed Res Int. 2018:9439182. DOI: 10.1155/2018/9439182. PMID: 29862299. PMCID: PMC5976919. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85047844769&origin=inward.28. De Luca A, Gallo M, Aldinucci D, Ribatti D, Lamura L, D'Alessio A, De Filippi R, Pinto A, Normanno N. 2011; Role of the EGFR ligand/receptor system in the secretion of angiogenic factors in mesenchymal stem cells. J Cell Physiol. 226:2131–2138. DOI: 10.1002/jcp.22548. PMID: 21520065. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79955096087&origin=inward.29. Maheshwari G, Wiley HS, Lauffenburger DA. 2001; Autocrine epidermal growth factor signaling stimulates directionally persistent mammary epithelial cell migration. J Cell Biol. 155:1123–1128. DOI: 10.1083/jcb.200109060. PMID: 11756466. PMCID: PMC2199328. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035945359&origin=inward.30. Kim YJ, Seo DH, Lee SH, Lee SH, An GH, Ahn HJ, Kwon D, Seo KW, Kang KS. 2018; Conditioned media from human umbilical cord blood-derived mesenchymal stem cells stimulate rejuvenation function in human skin. Biochem Biophys Rep. 16:96–102. DOI: 10.1016/j.bbrep.2018.10.007. PMID: 30417126. PMCID: PMC6205340. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85055317070&origin=inward.31. Yaish P, Gazit A, Gilon C, Levitzki A. 1988; Blocking of EGF-dependent cell proliferation by EGF receptor kinase inhibitors. Science. 242:933–935. DOI: 10.1126/science.3263702. PMID: 3263702. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0024240990&origin=inward.32. Heo JS, Lee YJ, Han HJ. 2006; EGF stimulates proliferation of mouse embryonic stem cells: involvement of Ca2+ influx and p44/42 MAPKs. Am J Physiol Cell Physiol. 290:C123–C133. DOI: 10.1152/ajpcell.00142.2005. PMID: 16107508. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33644828202&origin=inward.33. Wan J, Ramachandran R, Goldman D. 2012; HB-EGF is necessary and sufficient for Muller glia dedifferentiation and retina regeneration. Dev Cell. 22:334–347. DOI: 10.1016/j.devcel.2011.11.020. PMID: 22340497. PMCID: PMC3285435. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84857004538&origin=inward.34. Li X, Ye X, Qi J, Fan R, Gao X, Wu Y, Zhou L, Tong A, Guo G. 2016; EGF and curcumin co-encapsulated nanoparticle/hydrogel system as potent skin regeneration agent. Int J Nanomedicine. 11:3993–4009. DOI: 10.2147/IJN.S104350. PMID: 27574428. PMCID: PMC4993277. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84983353649&origin=inward.35. Shibata S, Maeda S, Kondo N, Chimura N, Inoue A, Fukata T. 2011; Identification of the signaling pathway of TNF-α-induced CCL17/TARC transcription in a canine keratinocyte cell line. Vet Immunol Immunopathol. 139:90–98. DOI: 10.1016/j.vetimm.2010.08.008. PMID: 20837364. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=78650894317&origin=inward.36. Choi JH, Kim HG, Jin SW, Han EH, Khanal T, Do MT, Hwang YP, Choi JM, Chun SS, Chung YC, Jeong TC, Jeong HG. 2013; Topical application of Pleurotus eryngii extracts inhibits 2,4-dinitrochlorobenzene-induced atopic dermatitis in NC/Nga mice by the regulation of Th1/Th2 balance. Food Chem Toxicol. 53:38–45. DOI: 10.1016/j.fct.2012.11.025. PMID: 23200891. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84871464276&origin=inward.37. Gandhi NA, Pirozzi G, Graham NMH. 2017; Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 13:425–437. DOI: 10.1080/1744666X.2017.1298443. PMID: 28277826. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85018324268&origin=inward.38. Jeong NH, Yang EJ, Jin M, Lee JY, Choi YA, Park PH, Lee SR, Kim SU, Shin TY, Kwon TK, Jang YH, Song KS, Kim SH. 2018; Esculetin from Fraxinus rhynchophylla attenuates atopic skin inflammation by inhibiting the expression of inflammatory cytokines. Int Immunopharmacol. 59:209–216. DOI: 10.1016/j.intimp.2018.04.005. PMID: 29656211. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85045418520&origin=inward.39. Park HH, Lee S, Yu Y, Yoo SM, Baek SY, Jung N, Seo KW, Kang KS. 2020; TGF-β secreted by human umbilical cord blood-derived mesenchymal stem cells ameliorates atopic dermatitis by inhibiting secretion of TNF-α and IgE. Stem Cells. 38:904–916. DOI: 10.1002/stem.3183. PMID: 32277785. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85083435033&origin=inward.40. Chen L, Tredget EE, Wu PY, Wu Y. 2008; Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 3:e1886. DOI: 10.1371/journal.pone.0001886. PMID: 18382669. PMCID: PMC2270908. PMID: 6b6f2edcba8b4e85983c024338f231bd. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=44849121129&origin=inward.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differentiation of Osteoblast Progenitor Cells from Human Umbilical Cord Blood
- Endothelial progenitor cells and mesenchymal stem cells from human cord blood
- Percutaneous transplantation of human umbilical cord-derived mesenchymal stem cells in a dog suspected to have fibrocartilaginous embolic myelopathy
- Difference in HLA-DR Expression of Human Umbilical Cord Blood Derived Mesenchymal Stem Cells after Tri-lineage Differentiation
- Comparative Evaluation for Potential Differentiation of Endothelial Progenitor Cells and Mesenchymal Stem Cells into Endothelial-Like Cells