J Korean Med Sci.
2020 Sep;35(38):e343. 10.3346/jkms.2020.35.e343.
COVID-19 Patients Upregulate Toll-like Receptor 4-mediated Inflammatory Signaling That Mimics Bacterial Sepsis
- Affiliations
-
- 1Division of Infectious Diseases, Department of Internal Medicine, Chungnam National University School of Medicine, Daejeon, Korea
- 2School of Biological Sciences and Technology, Chonnam National University, Gwangju, Korea
- 3Department of Microbiology, Chungnam National University School of Medicine, Daejeon, Korea
- 4Infection Control Convergence Research Center, Chungnam National University School of Medicine, Daejeon, Korea
- 5Division of Pulmonary and Critical Care, Department of Internal Medicine, Chungnam National University School of Medicine, Daejeon, Korea
- KMID: 2507100
- DOI: http://doi.org/10.3346/jkms.2020.35.e343
Abstract
- Background
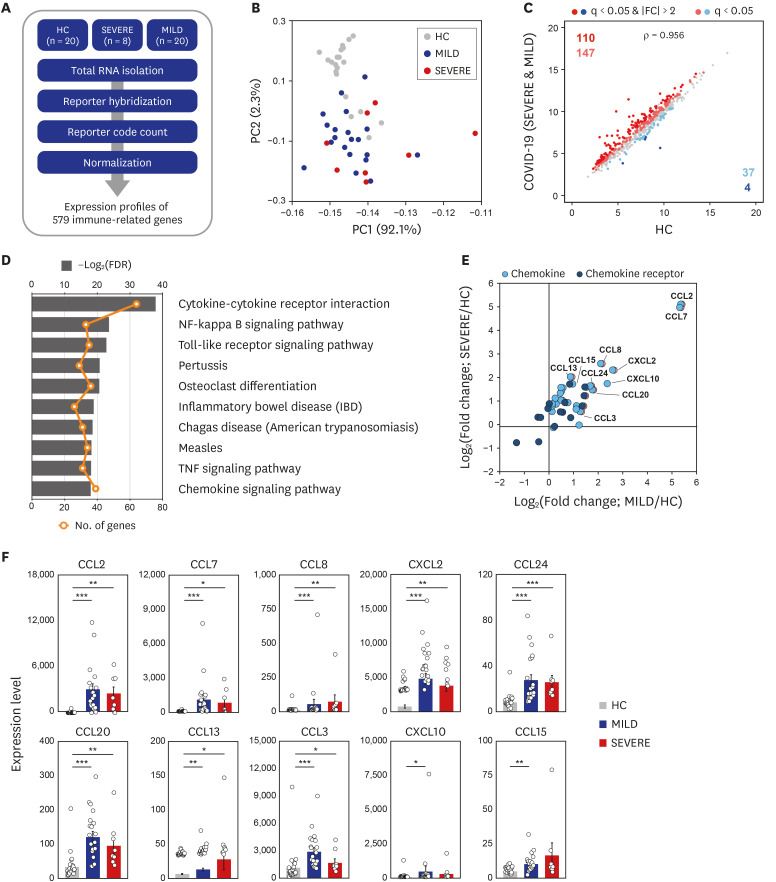
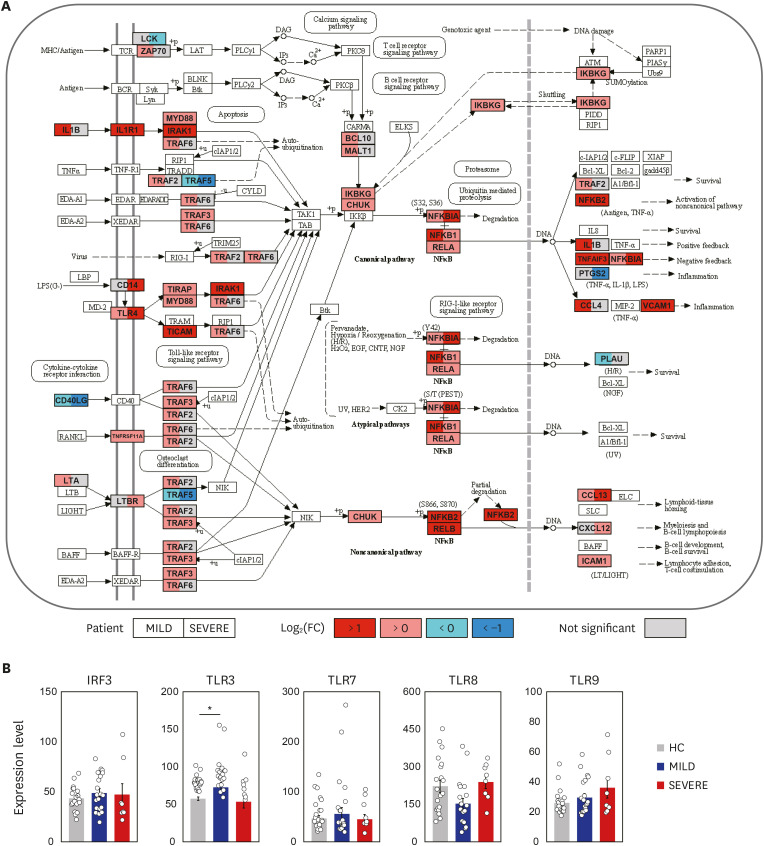
Observational studies of the ongoing coronavirus disease 2019 (COVID-19) outbreak suggest that a ‘cytokine storm’ is involved in the pathogenesis of severe illness. However, the molecular mechanisms underlying the altered pathological inflammation in COVID-19 are largely unknown. We report here that toll-like receptor (TLR) 4-mediated inflammatory signaling molecules are upregulated in peripheral blood mononuclear cells (PBMCs) from COVID-19 patients, compared with healthy controls (HC).
Methods
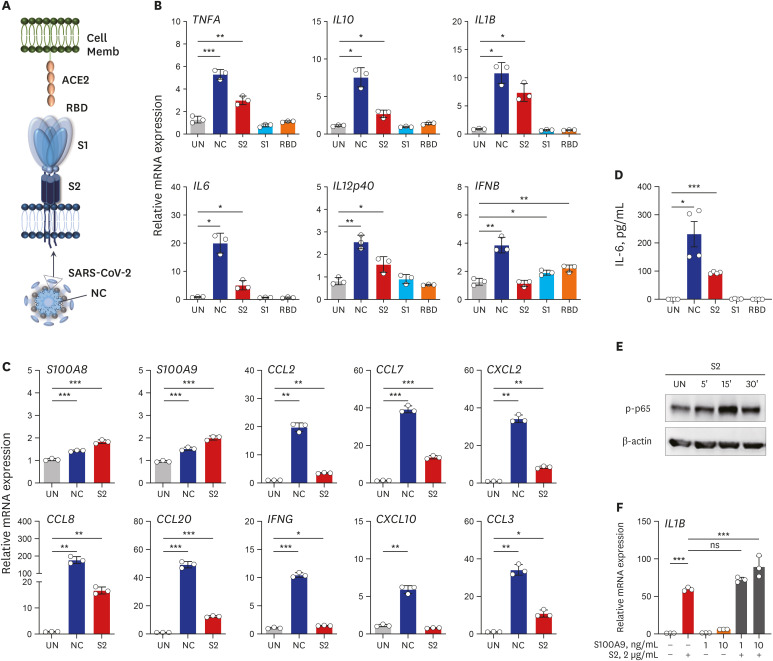
A total of 48 subjects including 28 COVID-19 patients (8 severe/critical vs. 20 mild/ moderate cases) admitted to Chungnam National University Hospital, and age/sex-matched 20 HC were enrolled in this study. PBMCs from the subjects were processed for nCounter Human Immunology gene expression assay to analyze the immune related transcriptome profiles. Recombinant proteins of severe acute respiratory syndrome coronavirus-2 (SARSCoV-2) were used to stimulate the PBMCs and monocyte-derived macrophages, and real-time polymerase chain reaction was performed to quantify the mRNA expressions of the proinflammatory cytokines/chemokines.
Results
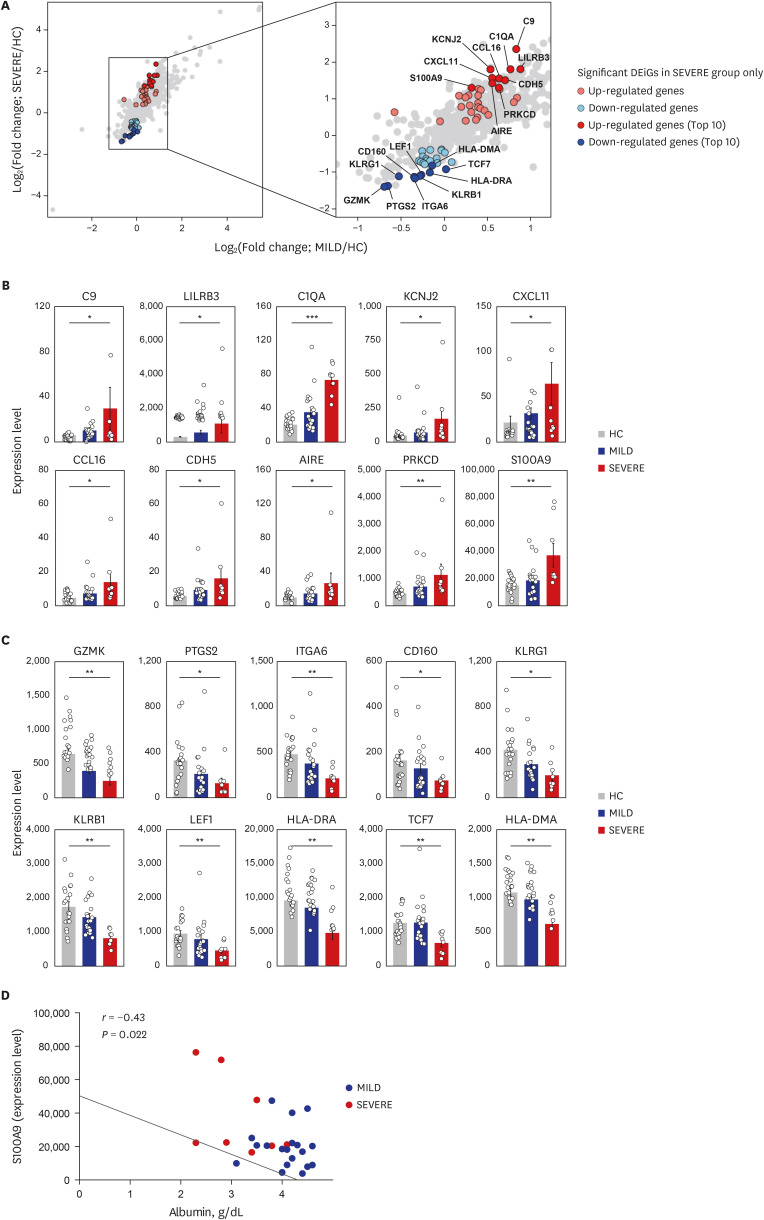
Among the most highly increased inflammatory mediators in severe/critically ill patients, S100A9, an alarmin and TLR4 ligand, was found as a noteworthy biomarker, because it inversely correlated with the serum albumin levels. We also observed that recombinant S2 and nucleocapsid proteins of SARS-CoV2 significantly increased proinflammatory cytokines/chemokines and S100A9 in human primary PBMCs.
Conclusion
These data support a link between TLR4 signaling and pathological inflammation during COVID-19 and contribute to develop therapeutic approaches through targeting TLR4-mediated inflammation.
Keyword
Figure
Reference
-
1. Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J, et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020; 201(11):1380–1388. PMID: 32275452.
Article2. Liang WH, Guan WJ, Li CC, Li YM, Liang HR, Zhao Y, et al. Clinical characteristics and outcomes of hospitalised patients with COVID-19 treated in Hubei (epicentre) and outside Hubei (non-epicentre): a nationwide analysis of China. Eur Respir J. 2020; 55(6):2000562. PMID: 32269086.
Article3. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020; 395(10229):1054–1062. PMID: 32171076.
Article4. World Health Organization. Coronavirus disease (COVID-19) pandemic. Updated 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.5. Azkur AK, Akdis M, Azkur D, Sokolowska M, van de Veen W, Brüggen MC, et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020; 75(7):1564–1581. PMID: 32396996.
Article6. Pascarella G, Strumia A, Piliego C, Bruno F, Del Buono R, Costa F, et al. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. 2020; 288(2):192–206. PMID: 32348588.
Article7. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020; 130(5):2620–2629. PMID: 32217835.
Article8. Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020; 94:91–95. PMID: 32173574.
Article9. Henry BM, de Oliveira MH, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020; 58(7):1021–1028. PMID: 32286245.
Article10. Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020; 10(2):102–108. PMID: 32282863.
Article11. Yang Y, Xiao Z, Ye K, He X, Sun B, Qin Z, et al. SARS-CoV-2: characteristics and current advances in research. Virol J. 2020; 17(1):117. PMID: 32727485.
Article12. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020; 181(2):281–292.e6. PMID: 32155444.
Article13. Bhatnager R, Bhasin M, Arora J, Dang AS. Epitope based peptide vaccine against SARS-CoV2: an immune-informatics approach. J Biomol Struct Dyn. 2020; 1–16.
Article14. Premkumar L, Segovia-Chumbez B, Jadi R, Martinez DR, Raut R, Markmann A, et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol. 2020; 5(48):eabc8413. PMID: 32527802.
Article15. McAndrews KM, Dowlatshahi DP, Dai J, Becker LM, Hensel J, Snowden LM, et al. Heterogeneous antibodies against SARS-CoV-2 spike receptor binding domain and nucleocapsid with implications on COVID-19 immunity. JCI Insight. 2020; 142386. PMID: 32796155.
Article16. World Health Organization. Clinical Management of COVID-19: Interim Guidance, 27 May 2020. Geneva: World Health Organization;2020.17. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395(10223):497–506. PMID: 31986264.
Article18. Kim JK, Lee HM, Park KS, Shin DM, Kim TS, Kim YS, et al. MIR144* inhibits antimicrobial responses against Mycobacterium tuberculosis in human monocytes and macrophages by targeting the autophagy protein DRAM2. Autophagy. 2017; 13(2):423–441. PMID: 27764573.19. Braschi B, Denny P, Gray K, Jones T, Seal R, Tweedie S, et al. Genenames.org: the HGNC and VGNC resources in 2019. Nucleic Acids Res. 2019; 47(D1):D786–92. PMID: 30304474.
Article20. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013; 310(20):2191–2194. PMID: 24141714.21. Hirano T, Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020; 52(5):731–733. PMID: 32325025.
Article22. Boo KH, Yang JS. Intrinsic cellular defenses against virus infection by antiviral type I interferon. Yonsei Med J. 2010; 51(1):9–17. PMID: 20046508.
Article23. Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Adv Exp Med Biol. 2005; 560:11–18. PMID: 15932016.
Article24. Kim ES, Chin BS, Kang CK, Kim NJ, Kang YM, Choi JP, et al. Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: a preliminary report of the first 28 patients from the Korean Cohort Study on COVID-19. J Korean Med Sci. 2020; 35(13):e142. PMID: 32242348.
Article25. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020; 395(10229):1033–1034. PMID: 32192578.
Article26. Mo P, Xing Y, Xiao Y, Deng L, Zhao Q, Wang H, et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. 2020; ciaa270. PMID: 32173725.
Article27. Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020; 71(15):762–768. PMID: 32161940.
Article28. Xiong Y, Liu Y, Cao L, Wang D, Guo M, Jiang A, et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect. 2020; 9(1):761–770. PMID: 32228226.
Article29. Lee JS, Park S, Jeong HW, Ahn JY, Choi SJ, Lee H, et al. Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci Immunol. 2020; 5(49):eabd1554. PMID: 32651212.
Article30. Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011; 30(1):16–34. PMID: 21235323.
Article31. Arpaia N, Barton GM. Toll-like receptors: key players in antiviral immunity. Curr Opin Virol. 2011; 1(6):447–454. PMID: 22440908.
Article32. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010; 11(5):373–384. PMID: 20404851.
Article33. Doyle SL, O'Neill LA. Toll-like receptors: from the discovery of NFκB to new insights into transcriptional regulations in innate immunity. Biochem Pharmacol. 2006; 72(9):1102–1113. PMID: 16930560.
Article34. Kawai T, Akira S. Signaling to NF-κB by Toll-like receptors. Trends Mol Med. 2007; 13(11):460–469. PMID: 18029230.
Article35. Ge Y, Huang M, Yao YM. Recent advances in the biology of IL-1 family cytokines and their potential roles in development of sepsis. Cytokine Growth Factor Rev. 2019; 45:24–34. PMID: 30587411.
Article36. Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013; 39(6):1003–1018. PMID: 24332029.
Article37. Chen JQ, Szodoray P, Zeher M. Toll-like receptor pathways in autoimmune diseases. Clin Rev Allergy Immunol. 2016; 50(1):1–17. PMID: 25687121.
Article38. Keogh B, Parker AE. Toll-like receptors as targets for immune disorders. Trends Pharmacol Sci. 2011; 32(7):435–442. PMID: 21529972.
Article39. Vogl T, Stratis A, Wixler V, Völler T, Thurainayagam S, Jorch SK, et al. Autoinhibitory regulation of S100A8/S100A9 alarmin activity locally restricts sterile inflammation. J Clin Invest. 2018; 128(5):1852–1866. PMID: 29611822.
Article40. Tomonobu N, Kinoshita R, Sakaguchi M. S100 soil sensor receptors and molecular targeting therapy against them in cancer metastasis. Transl Oncol. 2020; 13(4):100753. PMID: 32193075.
Article41. Nukui T, Ehama R, Sakaguchi M, Sonegawa H, Katagiri C, Hibino T, et al. S100A8/A9, a key mediator for positive feedback growth stimulation of normal human keratinocytes. J Cell Biochem. 2008; 104(2):453–464. PMID: 18044712.
Article42. Wang S, Song R, Wang Z, Jing Z, Wang S, Ma J. S100A8/A9 in Inflammation. Front Immunol. 2018; 9:1298. PMID: 29942307.
Article43. Leth-Larsen R, Zhong F, Chow VT, Holmskov U, Lu J. The SARS coronavirus spike glycoprotein is selectively recognized by lung surfactant protein D and activates macrophages. Immunobiology. 2007; 212(3):201–211. PMID: 17412287.
Article44. Guo G, Ye S, Xie S, Ye L, Lin C, Yang M, et al. The cytomegalovirus protein US31 induces inflammation through mono-macrophages in systemic lupus erythematosus by promoting NF-κB2 activation. Cell Death Dis. 2018; 9(2):104. PMID: 29367719.
Article45. Duette G, Pereyra Gerber P, Rubione J, Perez PS, Landay AL, Crowe SM, et al. Induction of HIF-1α by HIV-1 infection in CD4+ T cells promotes viral replication and drives extracellular vesicle-mediated inflammation. MBio. 2018; 9(5):e00757-18. PMID: 30206166.
Article46. RECOVERY Collaborative Group. Horby P, Lim WS, Emberson J, Mafham M, Bell J, et al. Dexamethasone in hospitalized patients with COVID-19 - preliminary report. N Engl J Med. 2020; NEJMoa2021436.
Article47. Bhattacharyya S, Zhao Y, Kay TW, Muglia LJ. Glucocorticoids target suppressor of cytokine signaling 1 (SOCS1) and type 1 interferons to regulate Toll-like receptor-induced STAT1 activation. Proc Natl Acad Sci U S A. 2011; 108(23):9554–9559. PMID: 21606371.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nucleic Acid Recognition and Signaling by Toll-like Receptor 9: Compartment-dependent Regulation
- Lipopolysaccharide: Basic Biochemistry, Intracellular Signaling, and Physiological Impacts in the Gut
- Peptidoglycan Induces the Production of Interleukin-8 via Calcium Signaling in Human Gingival Epithelium
- IGF-I Exerts an Anti-inflammatory Effect on Skeletal Muscle Cells through Down-regulation of TLR4 Signaling
- Bacterial 23S Ribosomal RNA, a Ligand for Toll-like Receptor 13