Cancer Res Treat.
2020 Jan;52(1):292-300. 10.4143/crt.2019.186.
Clinical Characteristics and Outcomes of Non-small Cell Lung Cancer Patients with HER2 Alterations in Korea
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Medicine, Dongsan Medical Center, Keimyung University School of Medicine, Daegu, Korea
- 2Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2501221
- DOI: http://doi.org/10.4143/crt.2019.186
Abstract
- Purpose
Human epidermal growth factor receptor 2 (HER2) alterations are found in approximately 1%-3% of non-small cell lung cancers (NSCLCs). We evaluated the clinical features and outcomes of NSCLC harboring HER2 alteration detected by next-generation sequencing (NGS) in Korea.
Materials and Methods
A total of 1,108 patients who were diagnosed with NSCLC between December 2015 and December 2017 were screened and analyzed by NGS. Medical records were reviewed retrospectively to analyze the clinical characteristics and outcomes from various treatments.
Results
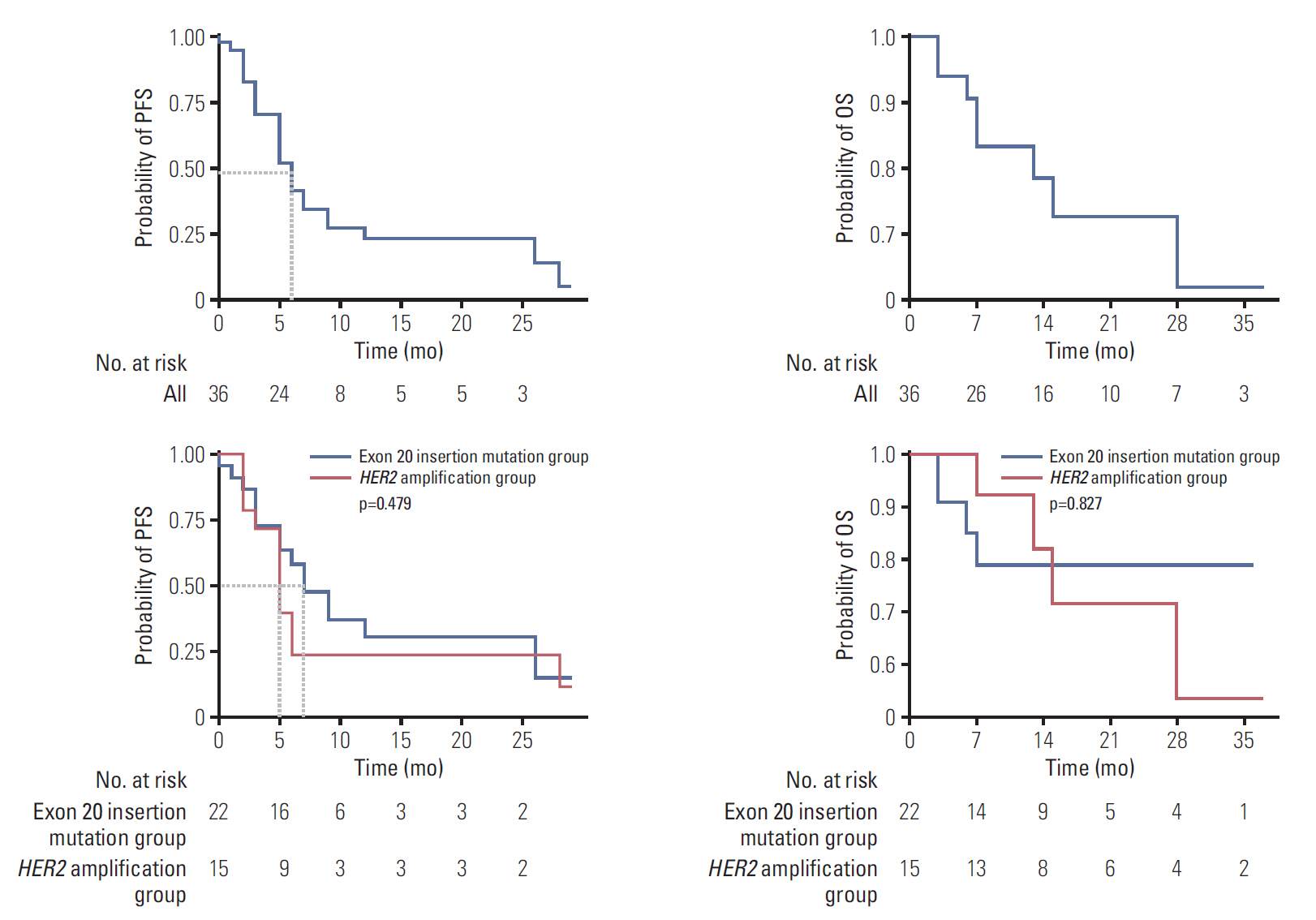
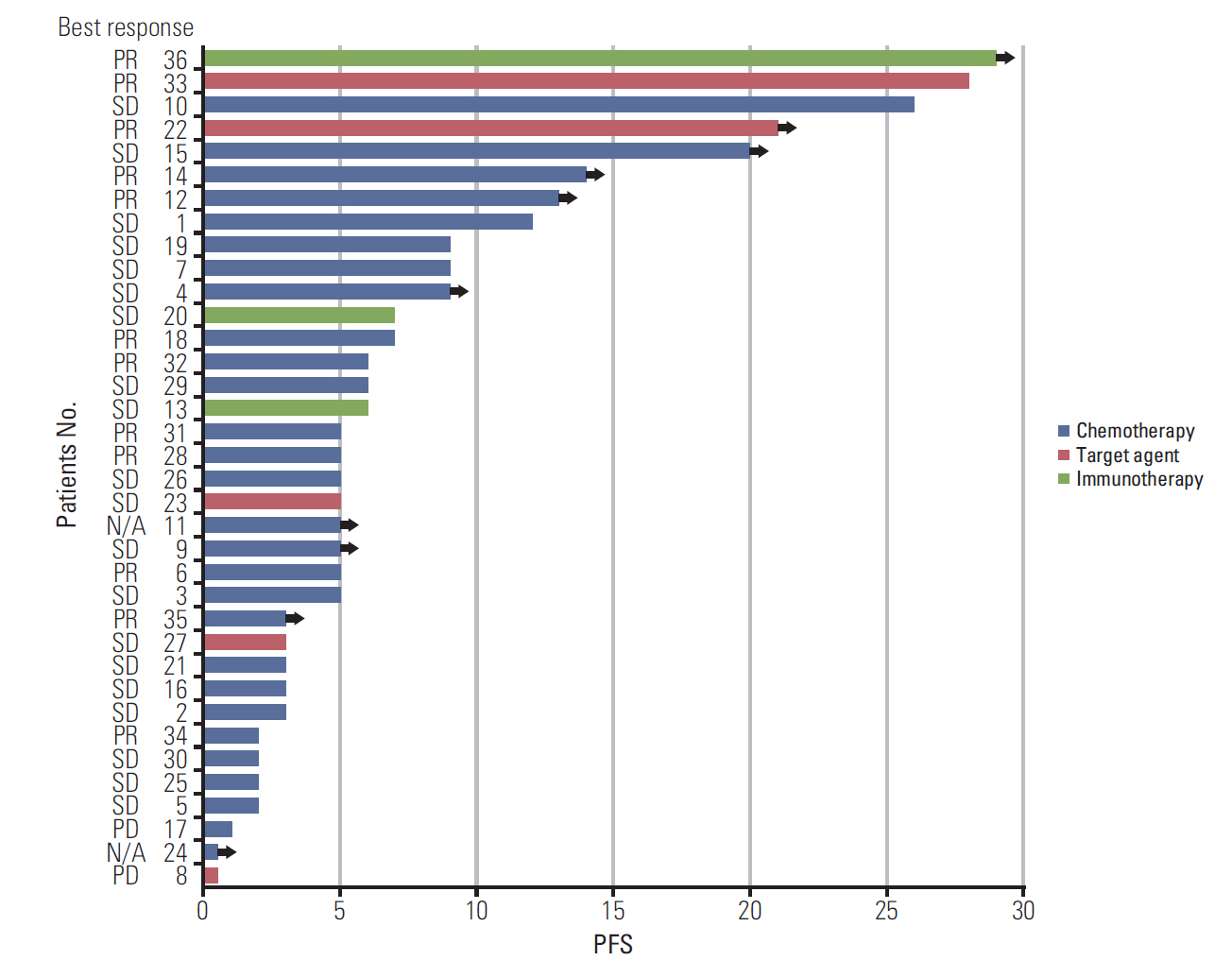
HER2 alterations were identified in 36 NSCLC patients. Of the patients, 22 (61.1%) had an exon 20 in-frame insertion mutation, 15 (41.7%) had HER2 amplification, and one had both. The median patient age was 58 years, 55.6% were male, and 50.0% were never-smokers. Adenocarcinoma was predominant (88.9%). The most common metastatic site was bone (58.3%), and 66.7% of patients were stage IV at initial diagnosis. Six patients (16.7%) had a coexistent sensitizing epidermal growth factor receptor (EGFR) mutation, and two patients (5.6%) had anaplastic lymphoma kinase (ALK) rearrangement. With a median 14 months of follow-up, the median progression-free survival of first-line treatment was 6 months (95% confidence interval, 4.172 to 7.828), and median overall survival was not reached. The proportions of adenocarcinoma, never-smokers, and metastasis to the liver were higher in the exon 20 in-frame insertion mutation group, whereas coexistence of EGFR mutation was more frequently found in the HER2 amplification group.
Conclusion
HER2-altered NSCLC showed distinct clinical features. Moreover, different characteristics were identified between the HER2 in-frame insertion mutation group and the HER2 amplification group.
Keyword
Figure
Cited by 1 articles
-
Real World Characteristics and Clinical Outcomes of
HER2 -Mutant Non–Small Cell Lung Cancer Patients Detected by Next-Generation Sequencing
Beung-Chul Ahn, Ye-Jeong Han, Hye Ryun Kim, Min Hee Hong, Byoung Chul Cho, Sun Min Lim
Cancer Res Treat. 2023;55(2):488-497. doi: 10.4143/crt.2022.359.
Reference
-
References
1. Shigematsu H, Takahashi T, Nomura M, Majmudar K, Suzuki M, Lee H, et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res. 2005; 65:1642–6.
Article2. Yu HA, Planchard D, Lovly CM. Sequencing therapy for genetically defined subgroups of non-small cell lung cancer. Am Soc Clin Oncol Educ Book. 2018; 38:726–39.
Article3. Mazieres J, Peters S, Lepage B, Cortot AB, Barlesi F, BeauFaller M, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol. 2013; 31:1997–2003.4. Li C, Sun Y, Fang R, Han X, Luo X, Wang R, et al. Lung adenocarcinomas with HER2-activating mutations are associated with distinct clinical features and HER2/EGFR copy number gains. J Thorac Oncol. 2012; 7:85–9.
Article5. Arcila ME, Chaft JE, Nafa K, Roy-Chowdhuri S, Lau C, Zaidinski M, et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res. 2012; 18:4910–8.
Article6. Pillai RN, Behera M, Berry LD, Rossi MR, Kris MG, Johnson BE, et al. HER2 mutations in lung adenocarcinomas: a report from the Lung Cancer Mutation Consortium. Cancer. 2017; 123:4099–105.
Article7. Shin HT, Choi YL, Yun JW, Kim NKD, Kim SY, Jeon HJ, et al. Prevalence and detection of low-allele-fraction variants in clinical cancer samples. Nat Commun. 2017; 8:1377.
Article8. Williams HL, Walsh K, Diamond A, Oniscu A, Deans ZC. Validation of the Oncomine(™) focus panel for next-generation sequencing of clinical tumour samples. Virchows Arch. 2018; 473:489–503.
Article9. Mazieres J, Barlesi F, Filleron T, Besse B, Monnet I, Beau-Faller M, et al. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: results from the European EUHER2 cohort. Ann Oncol. 2016; 27:281–6.10. Li BT, Ross DS, Aisner DL, Chaft JE, Hsu M, Kako SL, et al. HER2 amplification and HER2 mutation are distinct molecular targets in lung cancers. J Thorac Oncol. 2016; 11:414–9.
Article11. Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008; 26:3543–51.
Article12. Li BT, Shen R, Buonocore D, Olah ZT, Ni A, Ginsberg MS, et al. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: results from a phase II basket trial. J Clin Oncol. 2018; 36:2532–7.
Article13. Lai WC, Lebas L, Milia J, Barnes TA, Gautschi O, Peters S, et al. Afatinib in patients with metastatic HER2-mutant lung cancers: an international multicenter study. J Clin Oncol. 2017; 35(15 Suppl):9071.
Article14. Garrido-Castro AC, Felip E. HER2 driven non-small cell lung cancer (NSCLC): potential therapeutic approaches. Transl Lung Cancer Res. 2013; 2:122–7.15. Ogoshi Y. P3.13-35 Antitumor effect of neratinib targeting HER2-altered lung cancer. J Thorac Oncol. 2018; 13(10 Suppl):S990.
Article16. Chouitar J, Vincent S, Brake R, Li S. P2.13-32 TAK-788 is a novel and potent tyrosine kinase inhibitor with selective activity against EGFR/HER2. J Thorac Oncol. 2018; 13(10 Suppl):S811.
Article17. Liu S, Li S, Hai J, Wang X, Chen T, Quinn MM, et al. Targeting HER2 aberrations in non-small cell lung cancer with osimertinib. Clin Cancer Res. 2018; 24:2594–604.
Article18. Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013; 31:3327–34.
Article19. Wu YL, Zhou C, Hu CP, Feng JF, Lu S, Lu YH, et al. LUX-Lung 6: a randomized, open-label, phase III study of afatinib (A) versus gemcitabine/cisplatin (GC) as first-line treatment for Asian patients (pts) with EGFR mutation-positive (EGFR M+) advanced adenocarcinoma of the lung. J Clin Oncol. 2013; 31(15 Suppl):8016.
Article20. Fang W, Zhao S, Liang Y, Yang Y, Yang L, Dong X, et al. P088 Distribution of different HER2 mutations and their impact on clinical responses to afatinib in HER2-mutant lung cancer. J Thorac Oncol. 2018; 13(12 Suppl):S1080.21. Suzawa K, Toyooka S, Sakaguchi M, Morita M, Yamamoto H, Tomida S, et al. Antitumor effect of afatinib, as a human epidermal growth factor receptor 2-targeted therapy, in lung cancers harboring HER2 oncogene alterations. Cancer Sci. 2016; 107:45–52.22. Catania C, Passaro A, Rocco EG, Spitaleri G, Barberis M, Noberasco C, et al. Dramatic antitumor activity of nivolumab in advanced HER2-positive lung cancer. Clin Lung Cancer. 2016; 17:e179–83.
Article23. Yoshizawa A, Sumiyoshi S, Sonobe M, Kobayashi M, Uehara T, Fujimoto M, et al. HER2 status in lung adenocarcinoma: a comparison of immunohistochemistry, fluorescence in situ hybridization (FISH), dual-ISH, and gene mutations. Lung Cancer. 2014; 85:373–8.
Article24. Suzuki M, Shiraishi K, Yoshida A, Shimada Y, Suzuki K, Asamura H, et al. HER2 gene mutations in non-small cell lung carcinomas: concurrence with Her2 gene amplification and Her2 protein expression and phosphorylation. Lung Cancer. 2015; 87:14–22.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Druggable Targets of Squamous Cell Lung Cancer
- Alterations of the Apoptosis Genes and Their Products in Non-small Cell Lung Cancer Tissues
- Molecular Targeted Therapy in Lung Cancer
- Real World Characteristics and Clinical Outcomes of HER2-Mutant Non–Small Cell Lung Cancer Patients Detected by Next-Generation Sequencing
- Comparison of Clinicopathogenomic Features and Treatment Outcomes of EGFR and HER2 Exon 20 Insertion Mutations in Non–Small Cell Lung Cancer: Single-Institution Experience