Cancer Res Treat.
2024 Jul;56(3):774-784. 10.4143/crt.2023.1177.
Comparison of Clinicopathogenomic Features and Treatment Outcomes of EGFR and HER2 Exon 20 Insertion Mutations in Non–Small Cell Lung Cancer: Single-Institution Experience
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2557664
- DOI: http://doi.org/10.4143/crt.2023.1177
Abstract
- Purpose
Exon 20 insertion mutations (E20ins) in epidermal growth factor receptor (EGFR) or human epidermal growth factor receptor 2 (HER2) in non–small cell lung cancer (NSCLC) patients has become more important with emergence of novel agents targeting E20ins.
Materials and Methods
Advanced/Metastatic NSCLC patients with E20ins were included. EGFR E20ins was identified by two methods, next-generation sequencing (NGS) or real-time polymerase chain reaction (PCR), while HER2 E20ins was done by NGS only.
Results
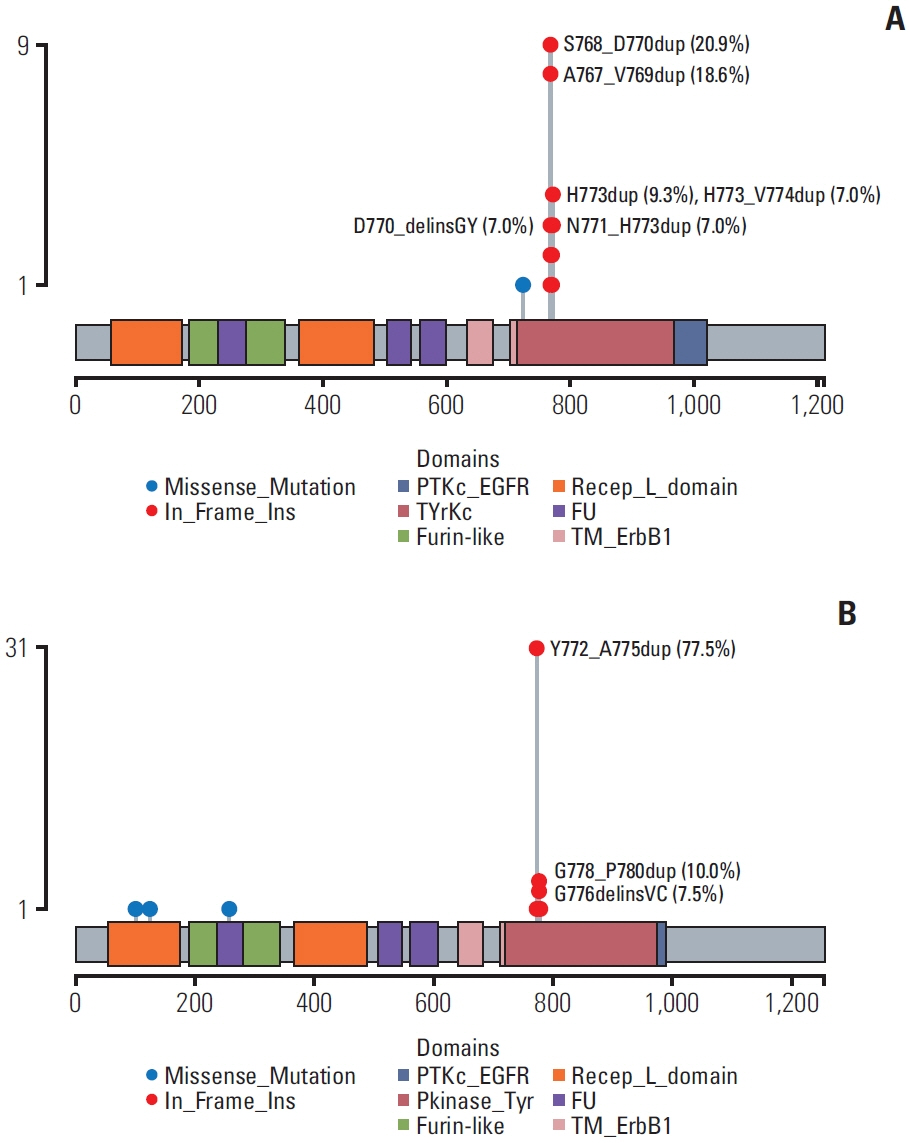
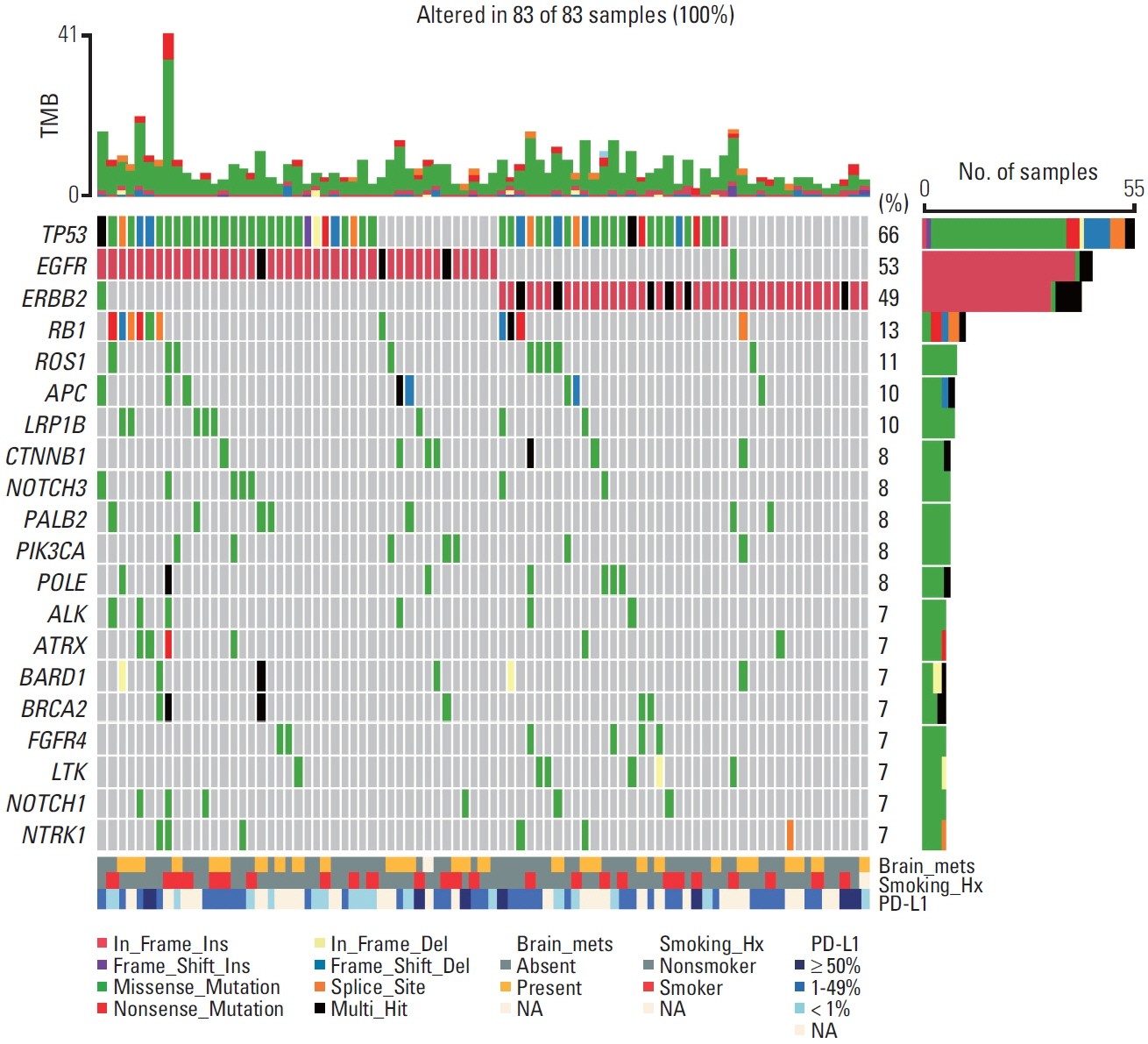
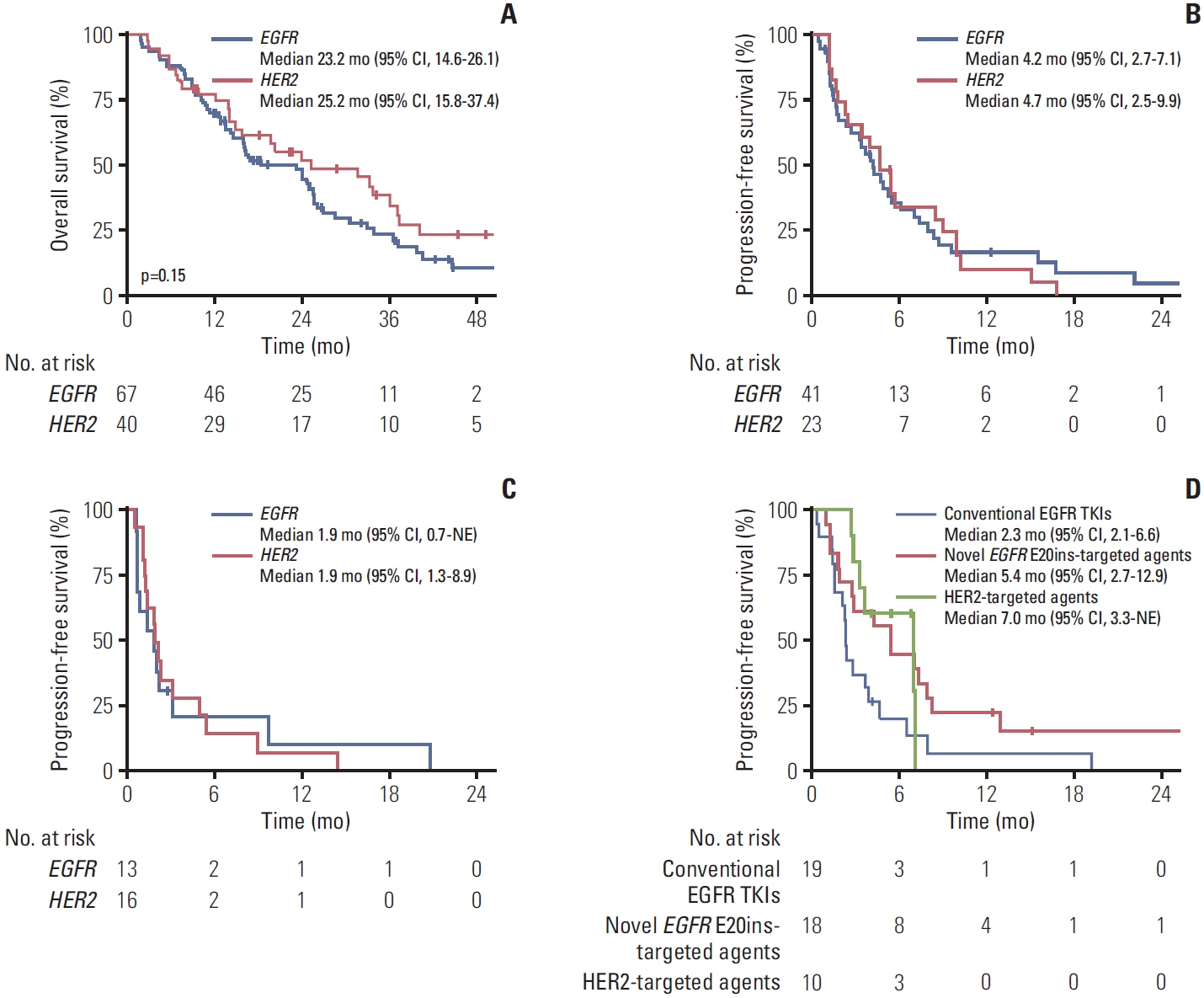
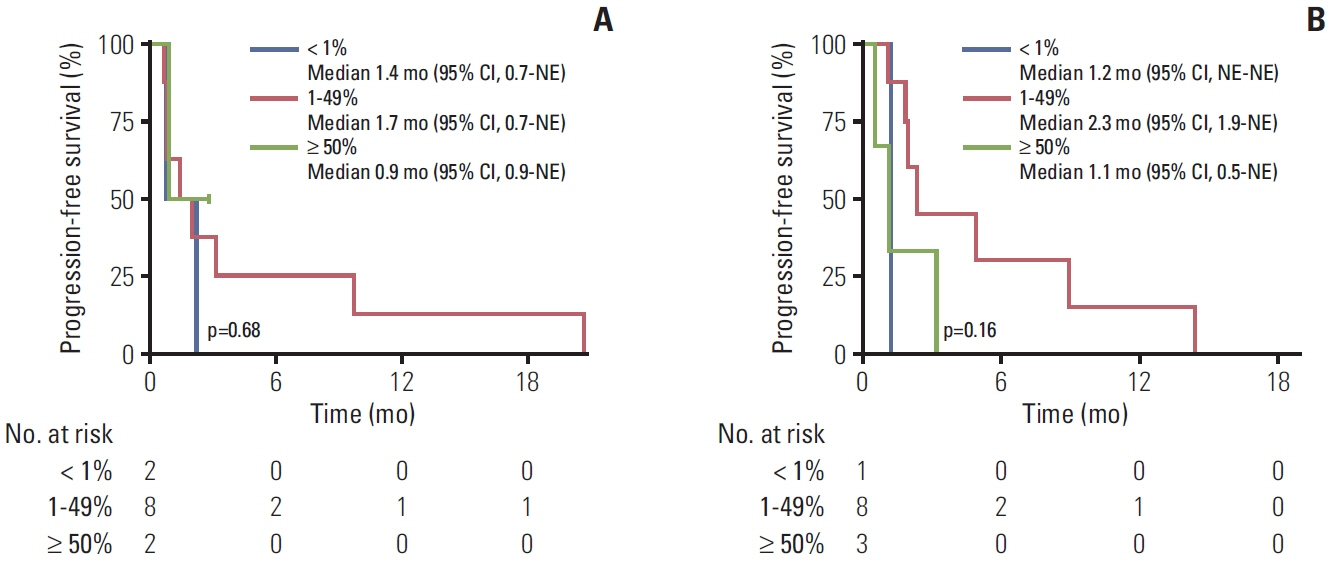
Between December 2013 and July 2021, E20ins were identified in 107 patients at Asan Medical Center; 67 EGFR E20ins and 40 HER2 E20ins. Out of 32 patients with EGFR E20ins who had tested both PCR and NGS, 17 were identified only through NGS and the other 15 through both tests, giving a discordance rate of 53.1%. There was no clinically significant difference in clinicopathologic features between EGFR and HER2 E20ins; both were observed more frequently in adenocarcinoma, female and never-smokers. Brain metastases were evident at diagnosis in 31.8% of EGFR E20ins and 27.5% of HER2 E20ins, respectively. Platinum-based doublets demonstrated objective response rates (ORR) of 13.3% with a median progression-free survival (PFS) of 4.2 months for EGFR E20ins and 35.3% with 4.7 months for HER2 E20ins, respectively. In contrast, novel EGFR E20ins-targeted agents exhibited an ORR of 46.2% with a median PFS of 5.4 months, while HER2-targeted agents showed an ORR of 50% with that of 7.0 months.
Conclusion
Identification of EGFR and HER2 E20ins is more important as their targeted therapies improved outcomes. Upfront NGS test as a comprehensive molecular approach is strongly warranted.
Figure
Reference
-
References
1. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012; 13:239–46.2. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014; 371:2167–77.
Article3. Shaw AT, Ou SH, Bang YJ, Camidge DR, Solomon BJ, Salgia R, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014; 371:1963–71.4. Yang JC, Ahn MJ, Kim DW, Ramalingam SS, Sequist LV, Su WC, et al. Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study phase II extension component. J Clin Oncol. 2017; 35:1288–96.
Article5. Planchard D, Besse B, Groen HJ, Souquet PJ, Quoix E, Baik CS, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol. 2016; 17:984–93.
Article6. Baraibar I, Mezquita L, Gil-Bazo I, Planchard D. Novel drugs targeting EGFR and HER2 exon 20 mutations in metastatic NSCLC. Crit Rev Oncol Hematol. 2020; 148:102906.
Article7. Beau-Faller M, Prim N, Ruppert AM, Nanni-Metellus I, Lacave R, Lacroix L, et al. Rare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer on 10 117 patients: a multicentre observational study by the French ERMETIC-IFCT network. Ann Oncol. 2014; 25:126–31.
Article8. Morita C, Yoshida T, Shirasawa M, Masuda K, Matsumoto Y, Shinno Y, et al. Clinical characteristics of advanced non-small cell lung cancer patients with EGFR exon 20 insertions. Sci Rep. 2021; 11:18762.9. Pillai RN, Behera M, Berry LD, Rossi MR, Kris MG, Johnson BE, et al. HER2 mutations in lung adenocarcinomas: a report from the Lung Cancer Mutation Consortium. Cancer. 2017; 123:4099–105.
Article10. Le X, Goldman JW, Clarke JM, Tchekmedyian N, Piotrowska Z, Chu D, et al. Poziotinib shows activity and durability of responses in subgroups of previously treated EGFR exon 20 NSCLC patients. J Clin Oncol. 2020; 38(15 Suppl):9514.
Article11. Park K, Haura EB, Leighl NB, Mitchell P, Shu CA, Girard N, et al. Amivantamab in EGFR exon 20 insertion-mutated non-small-cell lung cancer progressing on platinum chemotherapy: initial results from the CHRYSALIS phase I study. J Clin Oncol. 2021; 39:3391–402.12. Zhou C, Ramalingam SS, Kim TM, Kim SW, Yang JC, Riely GJ, et al. Treatment outcomes and safety of mobocertinib in platinum-pretreated patients with EGFR exon 20 insertionpositive metastatic non-small cell lung cancer: a phase 1/2 open-label nonrandomized clinical trial. JAMA Oncol. 2021; 7:e214761.13. Li BT, Smit EF, Goto Y, Nakagawa K, Udagawa H, Mazieres J, et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med. 2022; 386:241–51.14. Oxnard GR, Lo PC, Nishino M, Dahlberg SE, Lindeman NI, Butaney M, et al. Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. J Thorac Oncol. 2013; 8:179–84.
Article15. Arcila ME, Chaft JE, Nafa K, Roy-Chowdhuri S, Lau C, Zaidinski M, et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res. 2012; 18:4910–8.16. Russo A, Franchina T, Ricciardi G, Battaglia A, Picciotto M, Adamo V. Heterogeneous responses to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) in patients with uncommon EGFR mutations: new insights and future perspectives in this complex clinical scenario. Int J Mol Sci. 2019; 20:1431.
Article17. Bauml JM, Viteri S, Minchom A, Bazhenova L, Ou S, Schaffer M, et al. FP07.12 Uderdiagnosis of EGFR exon 20 insertion mutation variants: estimates from NGS-based Real-World Datasets. J Thorac Oncol. 2021; 16(3 Suppl):S208–9.18. Shah MP, Aredo JV, Padda SK, Ramchandran KJ, Wakelee HA, Das MS, et al. EGFR exon 20 insertion NSCLC and response to platinum-based chemotherapy. Clin Lung Cancer. 2022; 23:e148–53.19. Choudhury NJ, Schoenfeld AJ, Flynn J, Falcon CJ, Rizvi H, Rudin CM, et al. Response to standard therapies and comprehensive genomic analysis for patients with lung adenocarcinoma with EGFR exon 20 insertions. Clin Cancer Res. 2021; 27:2920–7.20. Gadgeel S, Rodriguez-Abreu D, Speranza G, Esteban E, Felip E, Domine M, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2020; 38:1505–17.
Article21. Kang EJ, Min KH, Hur GY, Lee SY, Shim JJ, Kang KH, et al. Comparison of the efficacy between pemetrexed plus platinum and non-pemetrexed plus platinum as first-line treatment in patients with wild-type epidermal growth factor receptor nonsquamous non-small cell lung cancer: a retrospective analysis. Chemotherapy. 2016; 61:41–50.
Article22. Metro G, Baglivo S, Bellezza G, Mandarano M, Gili A, Marchetti G, et al. Sensitivity to immune checkpoint blockade in advanced non-small cell lung cancer patients with EGFR exon 20 insertion mutations. Genes (Basel). 2021; 12:679.
Article23. Chen K, Pan G, Cheng G, Zhang F, Xu Y, Huang Z, et al. Immune microenvironment features and efficacy of PD-1/PD-L1 blockade in non-small cell lung cancer patients with EGFR or HER2 exon 20 insertions. Thorac Cancer. 2021; 12:218–26.24. Naidoo J, Sima CS, Rodriguez K, Busby N, Nafa K, Ladanyi M, et al. Epidermal growth factor receptor exon 20 insertions in advanced lung adenocarcinomas: clinical outcomes and response to erlotinib. Cancer. 2015; 121:3212–20.
Article25. Janssen announces U.S. FDA breakthrough therapy designation granted for JNJ-6372 for the treatment of non-small cell lung cancer [Internet]. New Brunswick, NJ: Johnson & Johnson; c2020 [cited 2023 May 4]. Available from: https://www.jnj.com/janssen-announces-u-s-fda-breakthrough-therapy-designation-granted-for-jnj-6372-for-the-treatment-of-non-small-cell-lung-cancer.26. Takeda announces U.S. FDA breakthrough therapy designation for mobocertinib (TAK-788) for the treatment of NSCLC patients with EGFR exon 20 insertion mutations [Internet]. Osaka: Takeda; c2020 [cited 2023 May 4]. Available from: https://www.takeda.com/newsroom/newsreleases/2020/takeda-announces-u.s.-fda-breakthrough-therapy-designation-for-mobocertinib-tak-788-for-the-treatment-of-nsclc-patients-with-egfr-exon-20-insertion-mutations.27. Agrawal T, Artis E, Xie J, Bhattacharya A, Haddish-Berhane N, Gopen T, et al. P76.74 PAPILLON: Randomized phase 3 study of amivantamab plus chemotherapy vs chemotherapy alone in EGFR exon20ins NSCLC. J Thorac Oncol. 2021; 16(3 Suppl):S621.
Article28. Sava J. FDA and Takeda to withdraw mobocertinib for EGFR Exon20+ NSCLC [Internet]. Cranbury, NJ: Targeted Oncology; c2023 [cited 2023 Dec 27]. Available from: https://www.targetedonc.com/view/fda-and-takeda-to-withdraw-mobocertinib-for-egfr-exon20-nsclc.29. Baek MY, Ahn HK, Park KR, Park HS, Kang SM, Park I, et al. Epidermal growth factor receptor mutation and pattern of brain metastasis in patients with non-small cell lung cancer. Korean J Intern Med. 2018; 33:168–75.
Article30. Riely GJ, Neal JW, Camidge DR, Spira AI, Piotrowska Z, Costa DB, et al. Activity and safety of mobocertinib (TAK-788) in previously treated non-small cell lung cancer with EGFR exon 20 insertion mutations from a phase I/II trial. Cancer Discov. 2021; 11:1688–99.31. Vyse S, Huang PH. Amivantamab for the treatment of EGFR exon 20 insertion mutant non-small cell lung cancer. Expert Rev Anticancer Ther. 2022; 22:3–16.
Article32. Vyse S, Huang PH. Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal Transduct Target Ther. 2019; 4:5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Outcomes of EGFR Exon 20 Insertion Mutations in Advanced Non-small Cell Lung Cancer in Korea
- Clinical Characteristics and Outcomes of Non-small Cell Lung Cancer Patients with HER2 Alterations in Korea
- A Case of Patient with Lung Adenocarcinoma with Double Rare EGFR Mutation of G719C and L861Q
- Does the efficacy of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor differ according to the type of EGFR mutation in non-small cell lung cancer?
- Clinical Features Reflect Exon Sites of EGFR Mutations in Patients with Resected Non-Small-Cell Lung Cancer