J Rheum Dis.
2020 Jan;27(1):51-60. 10.4078/jrd.2020.27.1.51.
Clinical Usefulness of Uric Acid as a Biomarker for Knee Osteoarthritis: A Comparative Analysis With Plain Radiography and Musculoskeletal Ultrasound
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Daegu Catholic University School of Medicine, Daegu, Korea. kimsk714@cu.ac.kr
- KMID: 2471962
- DOI: http://doi.org/10.4078/jrd.2020.27.1.51
Abstract
OBJECTIVE
The aim of this study was to determine the relationships of serum and urine uric acid with severity or activity in knee osteoarthritis (OA).
METHODS
A total of 42 patients with knee OA was enrolled, together with 58 healthy controls. Serum uric acid and spot urine uric acid levels were assessed for all patients. The severity and activity of knee OA were assessed by musculoskeletal ultrasound (MSUS) and plain radiography of the knee joint. Ultrasonographic abnormalities in knee OA includedsynovial hypertrophy, suprapatellar effusion, cartilage degradation, and osteophyte formation. Kellgren-Lawrence (K-L) grade was used to evaluate radiological progression of knee OA.
RESULTS
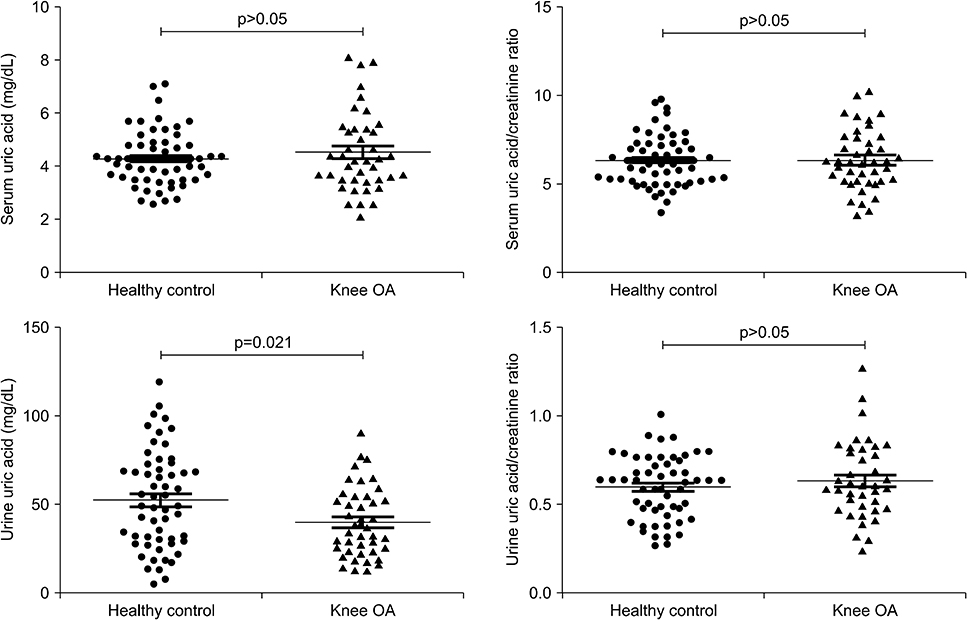
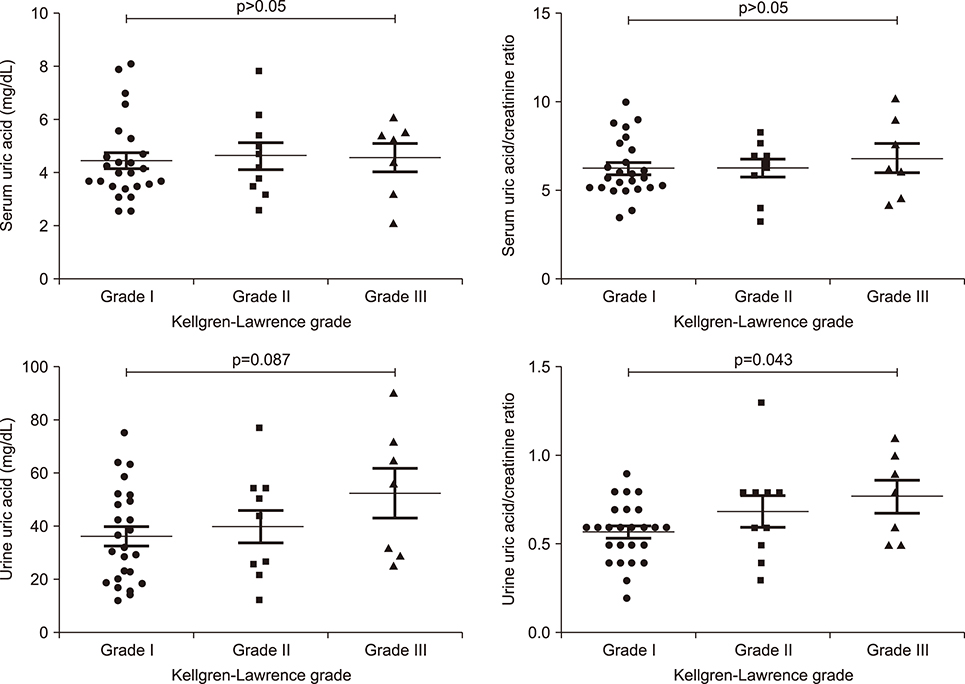
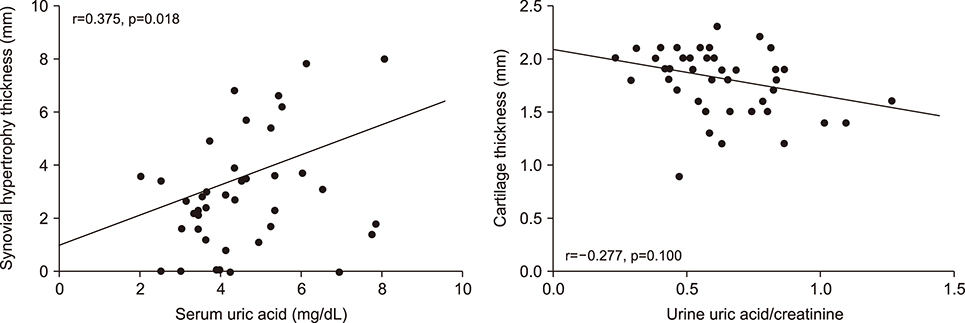
Patients with K-L grade III had a higher urine uric acid/creatinine ratio compared to those with K-L grade I (p=0.043). Patients with synovial hypertrophy had higher serum uric acid level compared to those without synovial hypertrophy (p=0.016). The urine uric acid/creatinine ratio was higher in patients with cartilage degradation compared to those without cartilage degradation (p=0.022). Serum uric acid was significantly associated with synovial hypertrophy thickness (r=0.375, p=0.018) but not with cartilage thickness after adjusting for age and body mass index. Lower urine uric acid was related with knee OA compared to healthy controls (odds ratio=0.974, 95% confidence interval 0.954~0.994, p=0.013).
CONCLUSION
The results of our study suggest that serum and urine uric acid reflects synovial inflammation based on MSUS and radiographic progression and then is associated with the pathogenesis of knee OA.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Ultrasound Findings were Associated With Radiographic Changes, But Not Clinical and Functional Outcomes in Hand Osteoarthritis
Seong-Kyu Kim, Ui Hong Jung, Ji-Won Kim, Jung-Yoon Choe
J Rheum Dis. 2021;28(1):17-24. doi: 10.4078/jrd.2021.28.1.17.Serum and Urine Uric Acid as a Biomarker in Osteoarthritis
Jae-Bum Jun
J Rheum Dis. 2020;27(2):75-77. doi: 10.4078/jrd.2020.27.2.75.
Reference
-
1. Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016; 12:412–420.
Article2. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019; 393:1745–1759.
Article3. van Spil WE, DeGroot J, Lems WF, Oostveen JC, Lafeber FP. Serum and urinary biochemical markers for knee and hip-osteoarthritis: a systematic review applying the consensus BIPED criteria. Osteoarthritis Cartilage. 2010; 18:605–612.
Article4. Lafeber FP, van Spil WE. Osteoarthritis year 2013 in review: biomarkers; reflecting before moving forward, one step at a time. Osteoarthritis Cartilage. 2013; 21:1452–1464.
Article5. Garnero P, Piperno M, Gineyts E, Christgau S, Delmas PD, Vignon E. Cross sectional evaluation of biochemical markers of bone, cartilage, and synovial tissue metabolism in patients with knee osteoarthritis: relations with disease activity and joint damage. Ann Rheum Dis. 2001; 60:619–626.
Article6. Hao HQ, Zhang JF, He QQ, Wang Z. Cartilage oligomeric matrix protein, C-terminal cross-linking telopeptide of type II collagen, and matrix metalloproteinase-3 as biomarkers for knee and hip osteoarthritis (OA) diagnosis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2019; 27:726–736.
Article7. Bardin T, Richette P. Impact of comorbidities on gout and hyperuricaemia: an update on prevalence and treatment options. BMC Med. 2017; 15:123.
Article8. Sun Y, Brenner H, Sauerland S, Günther KP, Puhl W, Stürmer T. Serum uric acid and patterns of radiographic osteoarthritis--the Ulm Osteoarthritis Study. Scand J Rheumatol. 2000; 29:380–386.9. Ding X, Zeng C, Wei J, Li H, Yang T, Zhang Y, et al. The associations of serum uric acid level and hyperuricemia with knee osteoarthritis. Rheumatol Int. 2016; 36:567–573.
Article10. Krasnokutsky S, Oshinsky C, Attur M, Ma S, Zhou H, Zheng F, et al. Serum urate levels predict joint space narrowing in non-gout patients with medial knee osteoarthritis. Arthritis Rheumatol. 2017; 69:1213–1220.
Article11. Denoble AE, Huffman KM, Stabler TV, Kelly SJ, Hershfield MS, McDaniel GE, et al. Uric acid is a danger signal of increasing risk for osteoarthritis through inflammasome activation. Proc Natl Acad Sci U S A. 2011; 108:2088–2093.
Article12. Vaidya B, Bhochhibhoya M, Nakarmi S. Synovial fluid uric acid level aids diagnosis of gout. Biomed Rep. 2018; 9:60–64.
Article13. Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Arthritis Rheum. 1986; 29:1039–1049.
Article14. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999; 130:461–470.
Article15. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957; 16:494–502.
Article16. Kan H, Arai Y, Kobayashi M, Nakagawa S, Inoue H, Hino M, et al. Radiographic measurement of joint space width using the fixed flexion view in 1,102 knees of Japanese patients with osteoarthritis in comparison with the standing extended view. Knee Surg Relat Res. 2017; 29:63–68.
Article17. Wakefield RJ, Balint PV, Szkudlarek M, Filippucci E, Backhaus M, D'Agostino MA, et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005; 32:2485–2487.18. Wu PT, Shao CJ, Wu KC, Wu TT, Chern TC, Kuo LC, et al. Pain in patients with equal radiographic grades of osteoarthritis in both knees: the value of gray scale ultrasound. Osteoarthritis Cartilage. 2012; 20:1507–1513.
Article19. Naredo E, Cabero F, Palop MJ, Collado P, Cruz A, Crespo M. Ultrasonographic findings in knee osteoarthritis: a comparative study with clinical and radiographic assessment. Osteoarthritis Cartilage. 2005; 13:568–574.
Article20. Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet. 2016; 388:2039–2052.
Article21. Zamudio-Cuevas Y, Martínez-Flores K, Fernández-Torres J, Loissell-Baltazar YA, Medina-Luna D, López-Macay A, et al. Monosodium urate crystals induce oxidative stress in human synoviocytes. Arthritis Res Ther. 2016; 18:117.
Article22. He Y, Hara H, Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016; 41:1012–1021.
Article23. Zheng SC, Zhu XX, Xue Y, Zhang LH, Zou HJ, Qiu JH, et al. Role of the NLRP3 inflammasome in the transient release of IL-1β induced by monosodium urate crystals in human fibroblast-like synoviocytes. J Inflamm (Lond). 2015; 12:30.
Article24. Puig JG, Torres RJ, de Miguel E, Sánchez A, Bailén R, Banegas JR. Uric acid excretion in healthy subjects: a nomogram to assess the mechanisms underlying purine metabolic disorders. Metabolism. 2012; 61:512–518.
Article25. Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V. Regulation of uric acid metabolism and excretion. Int J Cardiol. 2016; 213:8–14.
Article26. Kelton J, Kelley WN, Holmes EW. A rapid method for the diagnosis of acute uric acid nephropathy. Arch Intern Med. 1978; 138:612–615.
Article27. Choi S, Moon SJ, Kang EJ, Lee KH. Validity of random urinary uric acid-to-creatinine ratio for estimating 24-hour urine uric acid excretion in patients with gout. Ann Rheum Dis (Abstract). 2018; 77:664–665.28. Tiitinen S, Nissilä M, Ruutsalo HM, Isomäki H. Effect of nonsteroidal anti-inflammatory drugs on the renal excretion of uric acid. Clin Rheumatol. 1983; 2:233–236.
Article29. Chhana A, Callon KE, Pool B, Naot D, Gamble GD, Dray M, et al. The effects of monosodium urate monohydrate crystals on chondrocyte viability and function: implications for development of cartilage damage in gout. J Rheumatol. 2013; 40:2067–2074.
Article30. Hwang HS, Yang CM, Park SJ, Kim HA. Monosodium urate crystal-induced chondrocyte death via autophagic process. Int J Mol Sci. 2015; 16:29265–29277.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Clinical Usefulness of Uric Acid as a Biomarker for Knee Osteoarthritis: A Comparative Analysis With Plain Radiography and Musculoskeletal Ultrasound
- Application of Musculoskeletal Ultrasound in Osteoarthritis
- Serum and Urine Uric Acid as a Biomarker in Osteoarthritis
- Ultrasound Imaging Supplements the Plain Radiography in the Evaluation of the Knee Osteoarthritis
- The Uric Acid and Gout have No Direct Causality With Osteoarthritis: A Mendelian Randomization Study