J Breast Cancer.
2020 Feb;23(1):10-19. 10.4048/jbc.2020.23.e5.
Predicting the Benefit of Adjuvant Aromatase Inhibitor Therapy in Postmenopausal Breast Cancer Patients with Phosphorylated S6K1 Expression Status
- Affiliations
-
- 1Department of Surgery, Korea Cancer Center Hospital, Korea Institute of Radiological and Medical Sciences, Seoul, Korea. hyunah@kcch.re.kr
- 2Department of Surgery, National Medical Center, Seoul, Korea.
- 3Department of Pathology, Korea Cancer Center Hospital, Korea Institute of Radiological and Medical Sciences, Seoul, Korea.
- 4Division of Basic Radiation Bioscience, Korea Institute of Radiological and Medical Sciences, Seoul, Korea.
- KMID: 2470878
- DOI: http://doi.org/10.4048/jbc.2020.23.e5
Abstract
- PURPOSE
Phosphorylated ribosomal S6 kinase 1 (pS6K1) is a major downstream regulator of the mammalian target of rapamycin (mTOR) pathway. Recent studies have addressed the role of S6K1 in adipogenesis. pS6K1 may affect the outcome of estrogen depletion therapy in patients with hormone-sensitive breast cancer due to its association with adipogenesis and increased local estrogen levels. This study aimed to investigate the potential of pS6K1 as a predictive marker of adjuvant aromatase inhibitor (AI) therapy outcome in postmenopausal or ovarian function-suppressed patients with hormone-sensitive breast cancer.
METHODS
Medical records were retrospectively reviewed in postmenopausal or ovarian function-suppressed patients with estrogen receptor-positive and node-positive primary breast cancer. pS6K1 expression status was scored on a scale from 0 (negative) to 3+ (positive) based on immunohistochemical analysis.
RESULTS
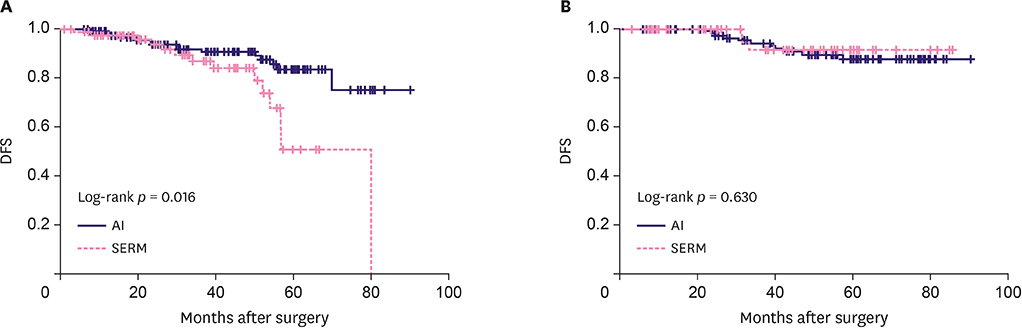
A total of 428 patients were eligible. The median follow-up duration was 44 months (range, 1-90). In patients with positive pS6K1 expression, AIs significantly improved disease-free survival (DFS) compared to selective estrogen receptor modulators (SERMs) (5 year-DFS: 83.5% vs. 50.7%, p = 0.016). However, there was no benefit of AIs on DFS in the pS6K1 negative group (5 year-DFS 87.6% vs. 91.4%, p = 0.630). On multivariate analysis, AI therapy remained a significant predictor for DFS in the pS6K1 positive group (hazard ratio, 0.39; 95% confidence interval, 0.16-0.96; p = 0.041). pS6K1 was more effective in predicting the benefit of AI therapy in patients with ages < 50 (p = 0.021) compared to those with ages ≥ 50 (p = 0.188).
CONCLUSION
pS6K1 expression may predict AI therapy outcomes and serve as a potential predictive marker for adjuvant endocrine therapy in postmenopausal and ovarian function-suppressed patients with hormone-sensitive breast cancer. AIs may be more effective in patients with pS6K1 positive tumors, while SERM could be considered an alternative option for patients with pS6K1 negative tumors.
Keyword
MeSH Terms
-
Adipogenesis
Aromatase Inhibitors
Aromatase*
Biomarkers, Tumor
Breast Neoplasms*
Breast*
Disease-Free Survival
Estrogens
Follow-Up Studies
Humans
Medical Records
Multivariate Analysis
Retrospective Studies
Ribosomal Protein S6 Kinases
Selective Estrogen Receptor Modulators
Sirolimus
Tamoxifen
Aromatase
Aromatase Inhibitors
Biomarkers, Tumor
Estrogens
Ribosomal Protein S6 Kinases
Selective Estrogen Receptor Modulators
Sirolimus
Tamoxifen
Figure
Reference
-
1. Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998; 351:1451–1467.2. Brodie AM, Njar VC. Aromatase inhibitors in advanced breast cancer: mechanism of action and clinical implications. J Steroid Biochem Mol Biol. 1998; 66:1–10.
Article3. Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005; 365:60–62.
Article4. Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, et al. Breast cancer, version 4.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018; 16:310–320.
Article5. Garreau JR, Delamelena T, Walts D, Karamlou K, Johnson N. Side effects of aromatase inhibitors versus tamoxifen: the patients' perspective. Am J Surg. 2006; 192:496–498.
Article6. Hadji P, Ziller V, Kyvernitakis J, Bauer M, Haas G, Schmidt N, et al. Persistence in patients with breast cancer treated with tamoxifen or aromatase inhibitors: a retrospective database analysis. Breast Cancer Res Treat. 2013; 138:185–191.
Article7. van der Hage JA, van den Broek LJ, Legrand C, Clahsen PC, Bosch CJ, Robanus-Maandag EC, et al. Overexpression of P70 S6 kinase protein is associated with increased risk of locoregional recurrence in node-negative premenopausal early breast cancer patients. Br J Cancer. 2004; 90:1543–1550.
Article8. Bärlund M, Forozan F, Kononen J, Bubendorf L, Chen Y, Bittner ML, et al. Detecting activation of ribosomal protein S6 kinase by complementary DNA and tissue microarray analysis. J Natl Cancer Inst. 2000; 92:1252–1259.
Article9. Yi SA, Han J, Han JW. Epigenetic role of nuclear S6K1 in early adipogenesis. BMB Rep. 2016; 49:401–402.
Article10. Yi SA, Um SH, Lee J, Yoo JH, Bang SY, Park EK, et al. S6K1 phosphorylation of H2B mediates EZH2 trimethylation of H3: a determinant of early adipogenesis. Mol Cell. 2016; 62:443–452.
Article11. Carnevalli LS, Masuda K, Frigerio F, Le Bacquer O, Um SH, Gandin V, et al. S6K1 plays a critical role in early adipocyte differentiation. Dev Cell. 2010; 18:763–774.
Article12. Bulun SE, Chen D, Moy I, Brooks DC, Zhao H. Aromatase, breast cancer and obesity: a complex interaction. Trends Endocrinol Metab. 2012; 23:83–89.
Article13. Cleary MP, Grossmann ME. Minireview: obesity and breast cancer: the estrogen connection. Endocrinology. 2009; 150:2537–2542.14. Kim EK, Kim HA, Koh JS, Kim MS, Kim KI, Lee JI, et al. Phosphorylated S6K1 is a possible marker for endocrine therapy resistance in hormone receptor-positive breast cancer. Breast Cancer Res Treat. 2011; 126:93–99.
Article15. Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014; 25:1901–1914.
Article16. Jiralerspong S, Goodwin PJ. Obesity and breast cancer prognosis: evidence, challenges, and opportunities. J Clin Oncol. 2016; 34:4203–4216.
Article17. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body fatness and cancer--viewpoint of the IARC Working Group. N Engl J Med. 2016; 375:794–798.
Article18. Paino F, La Noce M, Di Nucci D, Nicoletti GF, Salzillo R, De Rosa A, et al. Human adipose stem cell differentiation is highly affected by cancer cells both in vitro and in vivo: implication for autologous fat grafting. Cell Death Dis. 2017; 8:e2568.
Article19. Harada N. Aberrant expression of aromatase in breast cancer tissues. J Steroid Biochem Mol Biol. 1997; 61:175–184.
Article20. Zhao H, Zhou L, Shangguan AJ, Bulun SE. Aromatase expression and regulation in breast and endometrial cancer. J Mol Endocrinol. 2016; 57:R19–33.
Article21. Jiang N, Li Y, Shu T, Wang J. Cytokines and inflammation in adipogenesis: an updated review. Front Med. 2019; 13:314–329.
Article22. Weichhaus M, Broom I, Bermano G. The molecular contribution of TNF-α in the link between obesity and breast cancer. Oncol Rep. 2011; 25:477–483.
Article23. Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006; 6:184–192.
Article24. Azim HA, Kassem L, Treilleux I, Wang Q, El Enein MA, Anis SE, et al. Analysis of PI3K/mTOR pathway biomarkers and their prognostic value in women with hormone receptor-positive, HER2-negative early breast cancer. Transl Oncol. 2016; 9:114–123.
Article25. Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010; 11:1135–1141.
Article26. Breast International Group (BIG) 1-98 Collaborative Group. Thürlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005; 353:2747–2757.
Article27. Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat. 2012; 134:459–478.
Article28. Henry NL, Azzouz F, Desta Z, Li L, Nguyen AT, Lemler S, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol. 2012; 30:936–942.
Article29. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005; 365:1687–1717.30. Viale G, Regan MM, Dell'Orto P, Mastropasqua MG, Maiorano E, Rasmussen BB, et al. Which patients benefit most from adjuvant aromatase inhibitors? Results using a composite measure of prognostic risk in the BIG 1-98 randomized trial. Ann Oncol. 2011; 22:2201–2207.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Survival Benefit of Zoledronic Acid in Postmenopausal Breast Cancer Patients Receiving Aromatase Inhibitors
- Phosphorylated S6 Kinase-1 as Predictive Marker of Lapatinib Efficacy in Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer Patients
- Personalized therapy for advanced breast cancer using molecular signatures
- Effectiveness of bisphosphonate combined with activated vitamin D in patients with aromatase inhibitor-induced osteoporosis after breast cancer operation
- Effect of Vitamin D on Bone Mineral Density Changes in Patients with Breast Cancer Receiving Adjuvant Aromatase Inhibitor Therapy