J Breast Cancer.
2014 Dec;17(4):350-355. 10.4048/jbc.2014.17.4.350.
Survival Benefit of Zoledronic Acid in Postmenopausal Breast Cancer Patients Receiving Aromatase Inhibitors
- Affiliations
-
- 1Department of Surgery, Yonsei University College of Medicine, Seoul, Korea. gsjjoon@yuhs.ac
- 2Department of Surgery, Eulji University College of Medicine, Seoul, Korea.
- KMID: 2176126
- DOI: http://doi.org/10.4048/jbc.2014.17.4.350
Abstract
- PURPOSE
A growing body of evidence indicates that zoledronic acid (ZA) can improve the clinical outcome in patients with breast cancer and low estrogen levels. In the present study, we aimed to investigate the survival benefit of ZA administration in postmenopausal Korean women with breast cancer who were also receiving aromatase inhibitors.
METHODS
Between January 2004 and December 2010, 235 postmenopausal breast cancer patients undergoing aromatase inhibitor therapy were investigated. All patients were postmenopausal, as confirmed by laboratory tests. Of these patients, 77 received adjuvant upfront ZA for at least 1 year in addition to conventional adjuvant treatment. The remaining 158 patients never received ZA and were treated according to the St. Gallen guidelines.
RESULTS
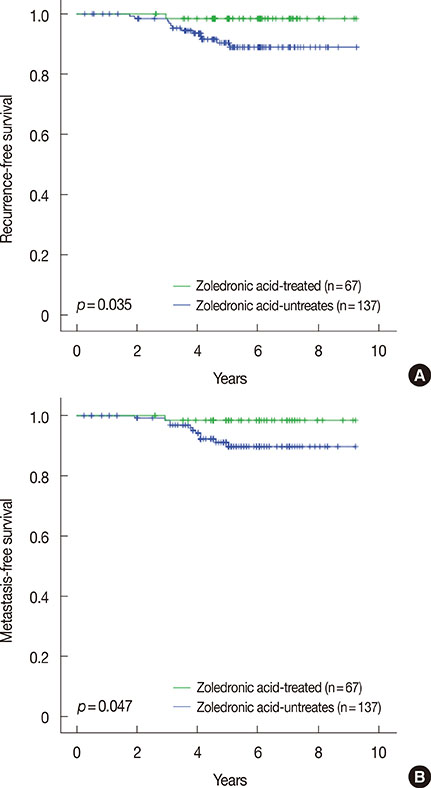
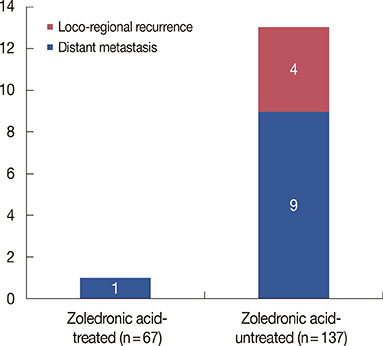
The baseline characteristics for ZA treatment were not different between the two groups. The median follow-up time was 62 months, and the patients who received ZA in addition to aromatase inhibitors showed a better recurrence-free survival compared to those who received aromatase inhibitors alone (p=0.035). On multivariate analysis, the patients who received ZA showed a better recurrence-free survival independent of the tumor size, nodal status, progesterone receptor, and histological grade. For this model, Harrell c index was 0.743. The hazard ratio of ZA use for recurrence-free survival was 0.12 (95% confidence interval, 0.01-0.99).
CONCLUSION
Our findings suggest that upfront use of ZA as part of adjuvant treatment can offer a survival benefit to postmenopausal breast cancer patients receiving aromatase inhibitor treatment.
MeSH Terms
Figure
Reference
-
1. Coleman RE, McCloskey EV. Bisphosphonates in oncology. Bone. 2011; 49:71–76.
Article2. Gnant M. Role of bisphosphonates in postmenopausal women with breast cancer. Cancer Treat Rev. 2014; 40:476–484.
Article3. Ibrahim A, Scher N, Williams G, Sridhara R, Li N, Chen G, et al. Approval summary for zoledronic acid for treatment of multiple myeloma and cancer bone metastases. Clin Cancer Res. 2003; 9:2394–2399.4. Bundred NJ, Campbell ID, Davidson N, DeBoer RH, Eidtmann H, Monnier A, et al. Effective inhibition of aromatase inhibitor-associated bone loss by zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: ZO-FAST Study results. Cancer. 2008; 112:1001–1010.
Article5. Coleman R, de Boer R, Eidtmann H, Llombart A, Davidson N, Neven P, et al. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann Oncol. 2013; 24:398–405.
Article6. Gnant MF, Mlineritsch B, Luschin-Ebengreuth G, Grampp S, Kaessmann H, Schmid M, et al. Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: a report from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol. 2007; 25:820–828.
Article7. Neville-Webbe HL, Coleman RE. Bisphosphonates and RANK ligand inhibitors for the treatment and prevention of metastatic bone disease. Eur J Cancer. 2010; 46:1211–1222.
Article8. Ottewell PD, Deux B, Mönkkönen H, Cross S, Coleman RE, Clezardin P, et al. Differential effect of doxorubicin and zoledronic acid on intraosseous versus extraosseous breast tumor growth in vivo. Clin Cancer Res. 2008; 14:4658–4666.
Article9. Ottewell PD, Mönkkönen H, Jones M, Lefley DV, Coleman RE, Holen I. Antitumor effects of doxorubicin followed by zoledronic acid in a mouse model of breast cancer. J Natl Cancer Inst. 2008; 100:1167–1178.
Article10. Jeong J, Lee KS, Choi YK, Oh YJ, Lee HD. Preventive effects of zoledronic acid on bone metastasis in mice injected with human breast cancer cells. J Korean Med Sci. 2011; 26:1569–1575.
Article11. Lipton A, Cook R, Saad F, Major P, Garnero P, Terpos E, et al. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer. 2008; 113:193–201.
Article12. Coleman RE, Marshall H, Cameron D, Dodwell D, Burkinshaw R, Keane M, et al. Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med. 2011; 365:1396–1405.
Article13. Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Pöstlberger S, Menzel C, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009; 360:679–691.
Article14. Lee SA, Hwang SH, Ahn SG, Lee HM, Jeong J, Lee HD. Effects of zoledronic acid on bone mineral density during aromatase inhibitor treatment of Korean postmenopausal breast cancer patients. Breast Cancer Res Treat. 2011; 130:863–870.
Article15. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998; 11:155–168.16. Moeder CB, Giltnane JM, Harigopal M, Molinaro A, Robinson A, Gelmon K, et al. Quantitative justification of the change from 10% to 30% for human epidermal growth factor receptor 2 scoring in the American Society of Clinical Oncology/College of American Pathologists guidelines: tumor heterogeneity in breast cancer and its implications for tissue microarray based assessment of outcome. J Clin Oncol. 2007; 25:5418–5425.
Article17. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996; 15:361–387.
Article18. Foroni C, Milan M, Strina C, Cappelletti M, Fumarola C, Bonelli M, et al. Pure anti-tumor effect of zoledronic acid in naive bone-only metastatic and locally advanced breast cancer: proof from the "biological window therapy". Breast Cancer Res Treat. 2014; 144:113–121.
Article19. Winter MC, Wilson C, Syddall SP, Cross SS, Evans A, Ingram CE, et al. Neoadjuvant chemotherapy with or without zoledronic acid in early breast cancer: a randomized biomarker pilot study. Clin Cancer Res. 2013; 19:2755–2765.
Article20. Fasching PA, Jud SM, Hauschild M, Kümmel S, Schütte M, Warm M, et al. FemZone trial: a randomized phase II trial comparing neoadjuvant letrozole and zoledronic acid with letrozole in primary breast cancer patients. BMC Cancer. 2014; 14:66.
Article21. Coleman RE, Winter MC, Cameron D, Bell R, Dodwell D, Keane MM, et al. The effects of adding zoledronic acid to neoadjuvant chemotherapy on tumour response: exploratory evidence for direct anti-tumour activity in breast cancer. Br J Cancer. 2010; 102:1099–1105.
Article22. Charehbili A, van de Ven S, Smit VT, Meershoek-Klein Kranenbarg E, Hamdy NA, Putter H, et al. Addition of zoledronic acid to neoadjuvant chemotherapy does not enhance tumor response in patients with HER2-negative stage II/III breast cancer: the NEOZOTAC trial (BOOG 2010-01). Ann Oncol. 2014; 25:998–1004.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bone-modifying agents for bone metastasis in patients with breast cancer
- Effectiveness of bisphosphonate combined with activated vitamin D in patients with aromatase inhibitor-induced osteoporosis after breast cancer operation
- Personalized therapy for advanced breast cancer using molecular signatures
- Preventive Effects of Zoledronic Acid on Bone Metastasis in Mice Injected with Human Breast Cancer Cells
- Predicting the Benefit of Adjuvant Aromatase Inhibitor Therapy in Postmenopausal Breast Cancer Patients with Phosphorylated S6K1 Expression Status