J Korean Med Sci.
2016 Sep;31(9):1373-1382. 10.3346/jkms.2016.31.9.1373.
Transplantation of Mesenchymal Stem Cells for Acute Spinal Cord Injury in Rats: Comparative Study between Intralesional Injection and Scaffold Based Transplantation
- Affiliations
-
- 1Department of Orthopaedic Surgery, The Catholic University of Korea, St. Vincent's Hospital, Suwon, Korea.
- 2Department of Orthopaedic Surgery, The Catholic University of Korea, Seoul St. Mary's Hospital, Seoul, Korea. kyh@catholic.ac.kr
- KMID: 2468268
- DOI: http://doi.org/10.3346/jkms.2016.31.9.1373
Abstract
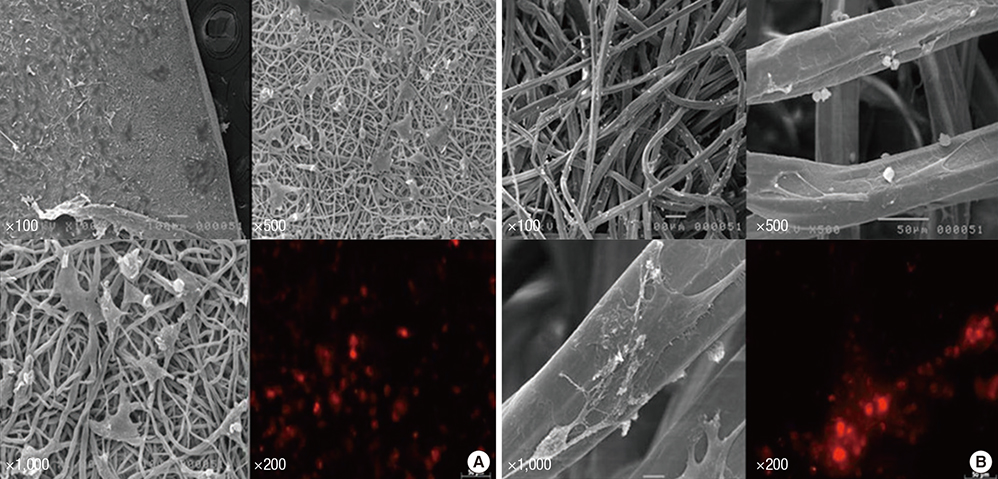
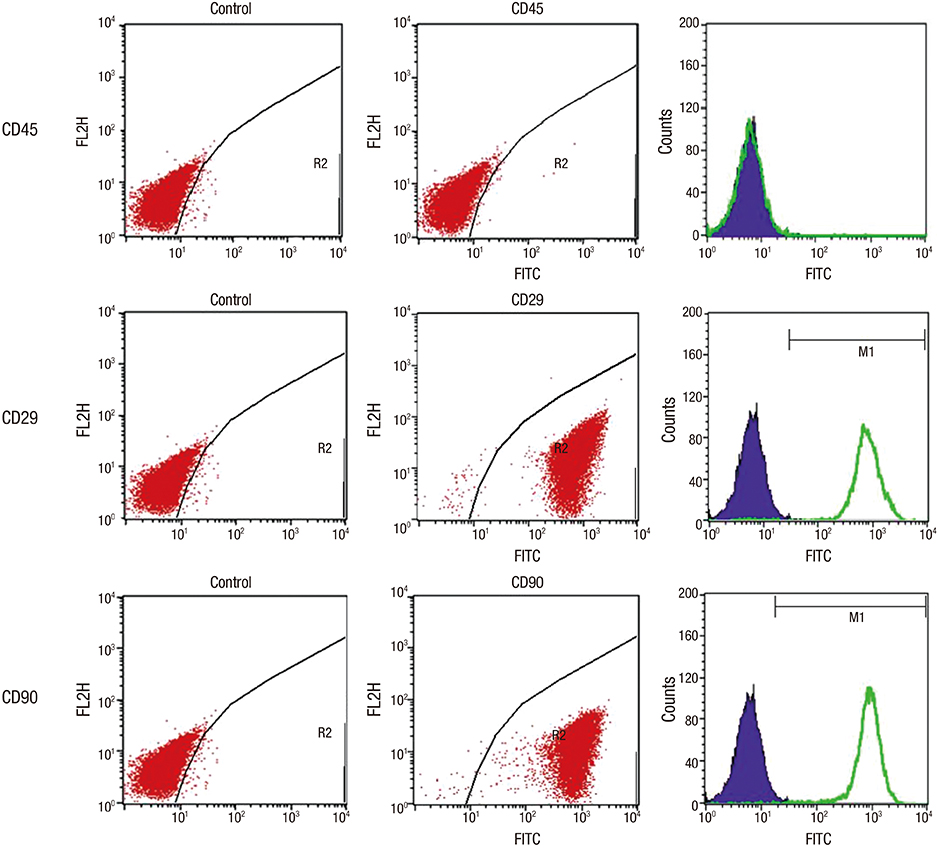
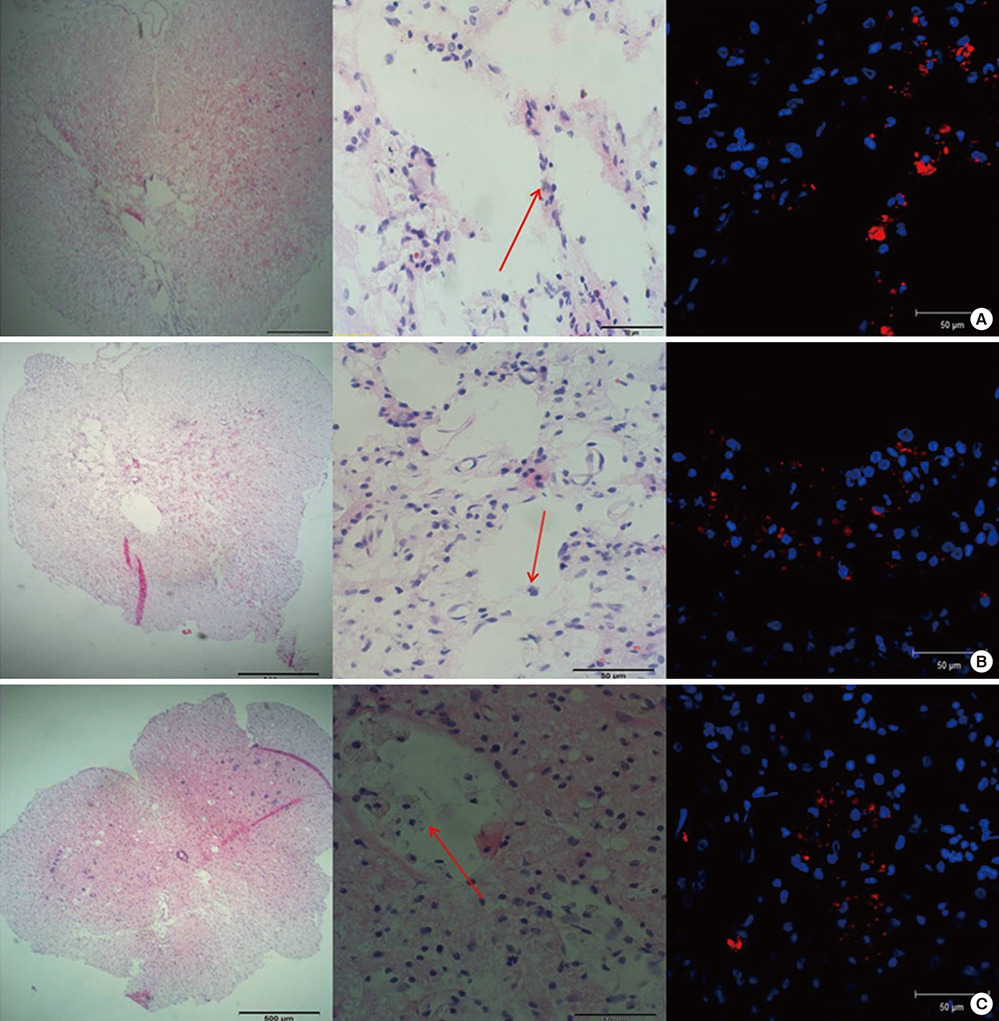
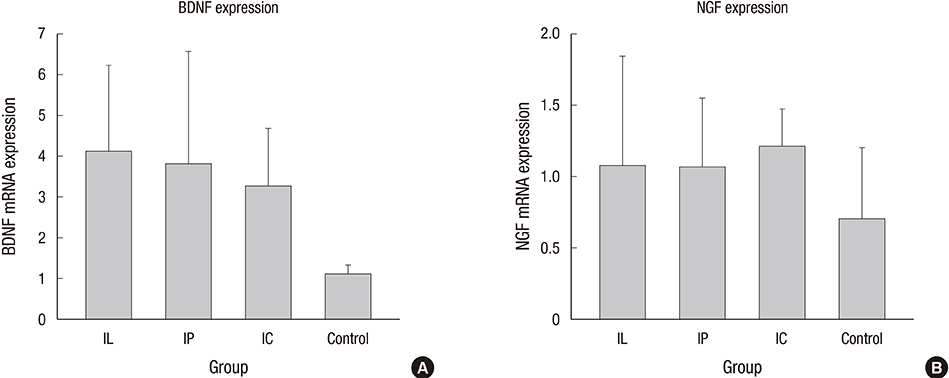
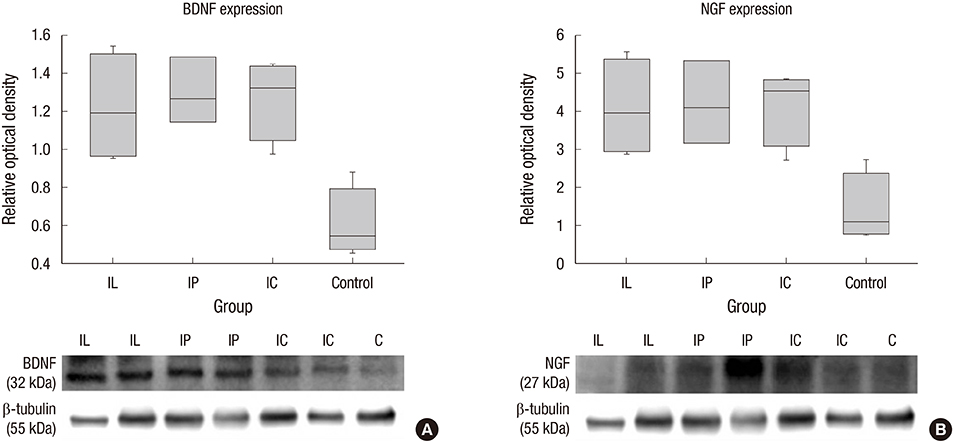
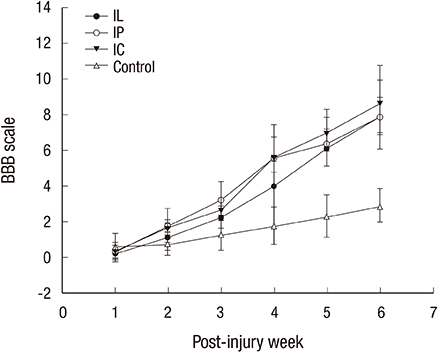
- Experimental stem cell therapy for spinal cord injury (SCI) has been extensively investigated. The selection of effective cell transplantation route is also an important issue. Although various types of scaffold have been widely tried as a carrier of stem cells to the injured spinal cord, there was little comparative study to investigate the efficacy of transplantation comparing with conventional transplantation route. A total of 48 Sprague-Dawley rats were subjected to standardized SCI, followed by transplantation of allogeneic mesenchymal stem cells (MSCs), either via intralesional injection (IL group), or via the poly (lactic-co-glycolic acid) (PLGA) scaffold (IP group) or chitosan scaffold (IC group). Engraftment and differentiation of the transplanted cells, expression of neurotrophic factors in the injured spinal cord, and functional recovery were compared with those of the control group. The mean numbers of engrafted MSCs in the IL, IP, and IC groups were 20.6 ± 0.7, 25.6 ± 1.7 and 26.7 ± 1.8 cells/high power filed (HPF), respectively. Results showed higher success rate of MSCs engraftment in the scaffold groups compared to the IL group. Expression of neuroprotective growth factors in the SCI lesions showed no significant differences between the IL, IP, and IC groups. The mean Basso, Beattie and Bresnahan locomotor scales at 6 weeks post-transplantation in the IL, IP, IC, and control groups were 7.9 ± 1.1, 7.9 ± 2.1, 8.7 ± 2.1, and 2.9 ± 1.0, respectively. The functional improvement was most excellent in the IC group. The scaffold based MSC transplantation for acute SCI presented the better cell engraftment and neuroprotective effect compared to the intralesional injection transplantation.
Keyword
MeSH Terms
-
Animals
Bone Marrow Cells/cytology
Brain-Derived Neurotrophic Factor/genetics/metabolism
Cell Differentiation
Cells, Cultured
Chitosan/chemistry
Immunophenotyping
Injections, Intralesional
Lactic Acid/chemistry
Male
*Mesenchymal Stem Cell Transplantation
Mesenchymal Stromal Cells/cytology
Microscopy, Fluorescence
Nerve Growth Factors/genetics/metabolism
Polyglycolic Acid/chemistry
Polylactic Acid-Polyglycolic Acid Copolymer
Rats
Rats, Sprague-Dawley
Real-Time Polymerase Chain Reaction
Recovery of Function
Spinal Cord/metabolism/pathology
Spinal Cord Injuries/metabolism/pathology/*therapy
*Tissue Scaffolds
Transplantation, Homologous
Brain-Derived Neurotrophic Factor
Nerve Growth Factors
Lactic Acid
Polylactic acid-polyglycolic acid copolymer
Polyglycolic Acid
Chitosan
Figure
Reference
-
1. Kamada T, Koda M, Dezawa M, Anahara R, Toyama Y, Yoshinaga K, Hashimoto M, Koshizuka S, Nishio Y, Mannoji C, et al. Transplantation of human bone marrow stromal cell-derived Schwann cells reduces cystic cavity and promotes functional recovery after contusion injury of adult rat spinal cord. Neuropathology. 2011; 31:48–58.2. Lu P, Jones LL, Tuszynski MH. BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exp Neurol. 2005; 191:344–360.3. Uccelli A, Benvenuto F, Laroni A, Giunti D. Neuroprotective features of mesenchymal stem cells. Best Pract Res Clin Haematol. 2011; 24:59–64.4. Chu K, Kim M, Park KI, Jeong SW, Park HK, Jung KH, Lee ST, Kang L, Lee K, Park DK, et al. Human neural stem cells improve sensorimotor deficits in the adult rat brain with experimental focal ischemia. Brain Res. 2004; 1016:145–153.5. Khang G, Kim HL, Hong M, Lee D. Neurogenesis of bone marrow-derived mesenchymal stem cells onto β-mercaptoethanol-loaded PLGA film. Cell Tissue Res. 2012; 347:713–724.6. Kang ES, Ha KY, Kim YH. Fate of transplanted bone marrow derived mesenchymal stem cells following spinal cord injury in rats by transplantation routes. J Korean Med Sci. 2012; 27:586–593.7. Kim JW, Ha KY, Molon JN, Kim YH. Bone marrow-derived mesenchymal stem cell transplantation for chronic spinal cord injury in rats: comparative study between intralesional and intravenous transplantation. Spine. 2013; 38:E1065–74.8. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995; 12:1–21.9. Cholas R, Hsu HP, Spector M. Collagen scaffolds incorporating select therapeutic agents to facilitate a reparative response in a standardized hemiresection defect in the rat spinal cord. Tissue Eng Part A. 2012; 18:2158–2172.10. Olson HE, Rooney GE, Gross L, Nesbitt JJ, Galvin KE, Knight A, Chen B, Yaszemski MJ, Windebank AJ. Neural stem cell- and Schwann cell-loaded biodegradable polymer scaffolds support axonal regeneration in the transected spinal cord. Tissue Eng Part A. 2009; 15:1797–1805.11. Paul C, Samdani AF, Betz RR, Fischer I, Neuhuber B. Grafting of human bone marrow stromal cells into spinal cord injury: a comparison of delivery methods. Spine. 2009; 34:328–334.12. de Haro J, Zurita M, Ayllón L, Vaquero J. Detection of 111In-oxine-labeled bone marrow stromal cells after intravenous or intralesional administration in chronic paraplegic rats. Neurosci Lett. 2005; 377:7–11.13. Chen X, Yang Y, Yao J, Lin W, Li Y, Chen Y, Gao Y, Yang Y, Gu X, Wang X. Bone marrow stromal cells-loaded chitosan conduits promote repair of complete transection injury in rat spinal cord. J Mater Sci Mater Med. 2011; 22:2347–2356.14. Buffo A, Rolando C, Ceruti S. Astrocytes in the damaged brain: molecular and cellular insights into their reactive response and healing potential. Biochem Pharmacol. 2010; 79:77–89.15. Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004; 24:2143–2155.16. Firkins SS, Bates CA, Stelzner DJ. Corticospinal tract plasticity and astroglial reactivity after cervical spinal injury in the postnatal rat. Exp Neurol. 1993; 120:1–15.17. Parr AM, Kulbatski I, Wang XH, Keating A, Tator CH. Fate of transplanted adult neural stem/progenitor cells and bone marrow-derived mesenchymal stromal cells in the injured adult rat spinal cord and impact on functional recovery. Surg Neurol. 2008; 70:600–607.18. Boido M, Garbossa D, Fontanella M, Ducati A, Vercelli A. Mesenchymal stem cell transplantation reduces glial cyst and improves functional outcome after spinal cord compression. World Neurosurg. 2014; 81:183–190.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of the Outcomes after Intralesional, Intracisternal, and Intravenous Transplantation of Human Bone Marrow Derived Mesenchymal Stem Cells for Spinal Cord Injured Rat
- Transplantation of Neural Stem Cells Cultured in Alginate Scaffold for Spinal Cord Injury in Rats
- Fate of Transplanted Bone Marrow Derived Mesenchymal Stem Cells Following Spinal Cord Injury in Rats by Transplantation Routes
- Clinical Response of 277 Patients with Spinal Cord Injury to Stem Cell Therapy in Iraq
- Clinical utilization of cord blood over human health: experience of stem cell transplantation and cell therapy using cord blood in Korea