Acute Crit Care.
2019 Nov;34(4):235-245. 10.4266/acc.2019.00717.
Fluid management in perioperative and critically ill patients
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, Korea. kyyoo@jnu.ac.kr
- KMID: 2467302
- DOI: http://doi.org/10.4266/acc.2019.00717
Abstract
- Fluid therapy to restore and/or maintain tissue perfusion may affect patient outcomes in perioperative, emergency, and intensive care. Kinetic analyses and outcome-oriented studies have provided more insight into fluid management. Crystalloids are slowly distributed to the interstitial space, and the efficiency (proportion of infused fluid retained in the bloodstream) is 50%−75% as long as infusion continues and may increase up to 100% when the arterial pressure has decreased. Elimination of the infused fluid during general anesthesia and surgery is very slow, amounting to only 10%-20% compared with that in conscious patients. When the endothelial glycocalyx layer is degraded in sepsis or trauma-induced systemic inflammation, turnover of colloids and crystalloids is accelerated and the efficiency is reduced, which may lead to tissue edema, inflammation, poor wound healing, and organ dysfunction. Balanced crystalloids are pragmatic initial resuscitation fluids and improve patient outcomes compared to saline (0.9% sodium chloride). Albumin may be beneficial, but other synthetic colloids appear to increase the risk of acute kidney injury and death among patients in the intensive care unit. Fluid kinetics is likely to change based on patient physiological conditions (e.g., general anesthesia, surgery, stress, dehydration, blood pressure, or inflammation) and fluid types. To maximize efficacy and minimize iatrogenic side effects, fluids should be prescribed based on individual patient factors, disease states, and other treatment remedies.
MeSH Terms
Figure
Cited by 1 articles
-
Principle of intravenous fluid therapy in the neurocritically ill patients
Sang-Bae Ko
J Neurocrit Care. 2024;17(2):41-48. doi: 10.18700/jnc.240044.
Reference
-
1. Connolly CM, Kramer GC, Hahn RG, Chaisson NF, Svensén CH, Kirschner RA, et al. Isoflurane but not mechanical ventilation promotes extravascular fluid accumulation during crystalloid volume loading. Anesthesiology. 2003; 98:670–81.
Article2. Zdolsek J, Li Y, Hahn RG. Detection of dehydration by using volume kinetics. Anesth Analg. 2012; 115:814–22.
Article3. Drobin D, Hahn RG. Volume kinetics of Ringer’s solution in hypovolemic volunteers. Anesthesiology. 1999; 90:81–91.
Article4. Hahn RG. Arterial pressure and the rate of elimination of crystalloid fluid. Anesth Analg. 2017; 124:1824–33.
Article5. Hahn RG, Lyons G. The half-life of infusion fluids: an educational review. Eur J Anaesthesiol. 2016; 33:475–82.6. Olsson J, Svensén CH, Hahn RG. The volume kinetics of acetated Ringer’s solution during laparoscopic cholecystectomy. Anesth Analg. 2004; 99:1854–60.
Article7. Hahn RG. Why are crystalloid and colloid fluid requirements similar during surgery and intensive care? Eur J Anaesthesiol. 2013; 30:515–8.
Article8. Hahn RG. Why crystalloids will do the job in the operating room. Anaesthesiol Intensive Ther. 2014; 46:342–9.
Article9. Hahn RG. Volume kinetics for infusion fluids. Anesthesiology. 2010; 113:470–81.
Article10. Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018; 378:829–39.
Article11. Self WH, Semler MW, Wanderer JP, Wang L, Byrne DW, Collins SP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018; 378:819–28.
Article12. Apfel CC, Meyer A, Orhan-Sungur M, Jalota L, Whelan RP, Jukar-Rao S. Supplemental intravenous crystalloids for the prevention of postoperative nausea and vomiting: quantitative review. Br J Anaesth. 2012; 108:893–902.
Article13. Holte K, Kehlet H. Compensatory fluid administration for preoperative dehydration: does it improve outcome? Acta Anaesthesiol Scand. 2002; 46:1089–93.14. Raghunathan K, Bonavia A, Nathanson BH, Beadles CA, Shaw AD, Brookhart MA, et al. Association between initial fluid choice and subsequent in-hospital mortality during the resuscitation of adults with septic shock. Anesthesiology. 2015; 123:1385–93.
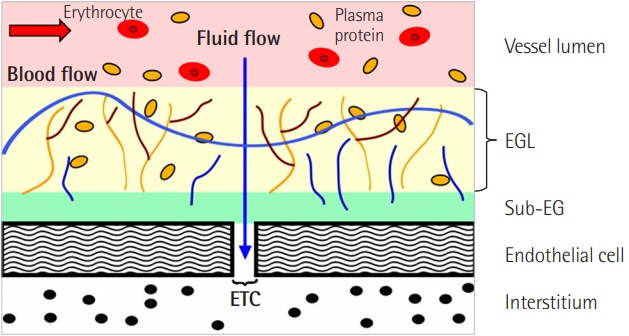
Article15. Alphonsus CS, Rodseth RN. The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia. 2014; 69:777–84.
Article16. Kolsen-Petersen JA. The endothelial glycocalyx: the great luminal barrier. Acta Anaesthesiol Scand. 2015; 59:137–9.
Article17. Chappell D, Jacob M. Role of the glycocalyx in fluid management: small things matter. Best Pract Res Clin Anaesthesiol. 2014; 28:227–34.
Article18. Uchimido R, Schmidt EP, Shapiro NI. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Crit Care. 2019; 23:16.
Article19. Fleck A, Raines G, Hawker F, Trotter J, Wallace PI, Ledingham IM, et al. Increased vascular permeability: a major cause of hypoalbuminaemia in disease and injury. Lancet. 1985; 1:781–4.
Article20. McFarlane C, Lee A. A comparison of Plasmalyte 148 and 0.9% saline for intra-operative fluid replacement. Anaesthesia. 1994; 49:779–81.
Article21. Scheingraber S, Rehm M, Sehmisch C, Finsterer U. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology. 1999; 90:1265–70.
Article22. McLean DJ, Shaw AD. Intravenous fluids: effects on renal outcomes. Br J Anaesth. 2018; 120:397–402.
Article23. Semler MW, Rice TW. Saline is not the first choice for crystalloid resuscitation fluids. Crit Care Med. 2016; 44:1541–4.
Article24. Yunos NM, Bellomo R, Glassford N, Sutcliffe H, Lam Q, Bailey M. Chloride-liberal vs. chloride-restrictive intravenous fluid administration and acute kidney injury: an extended analysis. Intensive Care Med. 2015; 41:257–64.
Article25. Boer C, Bossers SM, Koning NJ. Choice of fluid type: physiological concepts and perioperative indications. Br J Anaesth. 2018; 120:384–96.
Article26. Haase N, Perner A, Hennings LI, Siegemund M, Lauridsen B, Wetterslev M, et al. Hydroxyethyl starch 130/0.38-0.45 versus crystalloid or albumin in patients with sepsis: systematic review with meta-analysis and trial sequential analysis. BMJ. 2013; 346:f839.
Article27. Gattas DJ, Dan A, Myburgh J, Billot L, Lo S, Finfer S, et al. Fluid resuscitation with 6 % hydroxyethyl starch (130/0.4 and 130/0.42) in acutely ill patients: systematic review of effects on mortality and treatment with renal replacement therapy. Intensive Care Med. 2013; 39:558–68.28. Kammerer T, Brettner F, Hilferink S, Hulde N, Klug F, Pagel JI, et al. No differences in renal function between balanced 6% hydroxyethyl starch (130/0.4) and 5% albumin for volume replacement therapy in patients undergoing cystectomy: a randomized controlled trial. Anesthesiology. 2018; 128:67–78.29. Kabon B, Sessler DI, Kurz A, Crystalloid-Colloid Study Team. Effect of intraoperative goal-directed balanced crystalloid versus colloid administration on major postoperative morbidity: a randomized trial. Anesthesiology. 2019; 130:728–44.30. Rehm M, Haller M, Orth V, Kreimeier U, Jacob M, Dressel H, et al. Changes in blood volume and hematocrit during acute preoperative volume loading with 5% albumin or 6% hetastarch solutions in patients before radical hysterectomy. Anesthesiology. 2001; 95:849–56.
Article31. Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res. 2010; 87:198–210.
Article32. Starling EH. On the absorption of fluids from the connective tissue spaces. J Physiol. 1896; 19:312–26.
Article33. Adamson RH, Lenz JF, Zhang X, Adamson GN, Weinbaum S, Curry FE. Oncotic pressures opposing filtration across nonfenestrated rat microvessels. J Physiol. 2004; 557(Pt 3):889–907.
Article34. Woodcock TE, Woodcock TM. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. Br J Anaesth. 2012; 108:384–94.
Article35. Hall JE. The body fluid compartments: extracellular and intracellular fluids; edema. In : Hall JE, editor. Guyton and Hall textbook of medical physiology. 13th ed. Philadelphia: Elsevier;2016. p. 305–21.36. Drobin D, Hahn RG. Kinetics of isotonic and hypertonic plasma volume expanders. Anesthesiology. 2002; 96:1371–80.
Article37. Reid F, Lobo DN, Williams RN, Rowlands BJ, Allison SP. (Ab) normal saline and physiological Hartmann’s solution: a randomized double-blind crossover study. Clin Sci (Lond). 2003; 104:17–24.38. Sjöstrand F, Edsberg L, Hahn RG. Volume kinetics of glucose solutions given by intravenous infusion. Br J Anaesth. 2001; 87:834–43.
Article39. Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983; 71:726–35.
Article40. Hahn RG. The elimination half-life of crystalloid fluid is shorter in female than in male volunteers: a retrospective population kinetic analysis. Biol Sex Differ. 2016; 7:54.
Article41. Lobo DN, Stanga Z, Aloysius MM, Wicks C, Nunes QM, Ingram KL, et al. Effect of volume loading with 1 liter intravenous infusions of 0.9% saline, 4% succinylated gelatine (Gelofusine) and 6% hydroxyethyl starch (Voluven) on blood volume and endocrine responses: a randomized, three-way crossover study in healthy volunteers. Crit Care Med. 2010; 38:464–70.
Article42. Holte K, Hahn RG, Ravn L, Bertelsen KG, Hansen S, Kehlet H. Influence of “liberal” versus “restrictive” intraoperative fluid administration on elimination of a postoperative fluid load. Anesthesiology. 2007; 106:75–9.43. Brandstrup B, Tønnesen H, Beier-Holgersen R, Hjortsø E, Ørding H, Lindorff-Larsen K, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003; 238:641–8.44. Hahn RG, Drobin D, Zdolsek J. Distribution of crystalloid fluid changes with the rate of infusion: a population-based study. Acta Anaesthesiol Scand. 2016; 60:569–78.
Article45. Hahn RG, Lindahl CC, Drobin D. Volume kinetics of acetated Ringer’s solution during experimental spinal anaesthesia. Acta Anaesthesiol Scand. 2011; 55:987–94.
Article46. Ewaldsson CA, Hahn RG. Volume kinetics of Ringer’s solution during induction of spinal and general anaesthesia. Br J Anaesth. 2001; 87:406–14.
Article47. Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012; 256:18–24.
Article48. Shaw AD, Bagshaw SM, Goldstein SL, Scherer LA, Duan M, Schermer CR, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma-Lyte. Ann Surg. 2012; 255:821–9.49. Chua HR, Venkatesh B, Stachowski E, Schneider AG, Perkins K, Ladanyi S, et al. Plasma-Lyte 148 vs 0.9% saline for fluid resuscitation in diabetic ketoacidosis. J Crit Care. 2012; 27:138–45.
Article50. Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017; 43:304–77.51. Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008; 358:125–39.
Article52. Joosten A, Delaporte A, Ickx B, Touihri K, Stany I, Barvais L, et al. Crystalloid versus colloid for intraoperative goal-directed fluid therapy using a closed-loop system: a randomized, double-blinded, controlled trial in major abdominal surgery. Anesthesiology. 2018; 128:55–66.53. Hahn RG. Adverse effects of crystalloid and colloid fluids. Anaesthesiol Intensive Ther. 2017; 49:303–8.
Article54. Reuter DA, Chappell D, Perel A. The dark sides of fluid administration in the critically ill patient. Intensive Care Med. 2018; 44:1138–40.
Article55. Vincent JL. Fluid management in the critically ill. Kidney Int. 2019; 96:52–7.
Article56. Brandstrup B, Svensen C, Engquist A. Hemorrhage and operation cause a contraction of the extracellular space needing replacement: evidence and implications?: a systematic review. Surgery. 2006; 139:419–32.57. Varadhan KK, Lobo DN. A meta-analysis of randomised controlled trials of intravenous fluid therapy in major elective open abdominal surgery: getting the balance right. Proc Nutr Soc. 2010; 69:488–98.
Article58. Ewaldsson CA, Hahn RG. Kinetics and extravascular retention of acetated ringer’s solution during isoflurane or propofol anesthesia for thyroid surgery. Anesthesiology. 2005; 103:460–9.
Article59. Glatz T, Kulemann B, Marjanovic G, Bregenzer S, Makowiec F, Hoeppner J. Postoperative fluid overload is a risk factor for adverse surgical outcome in patients undergoing esophagectomy for esophageal cancer: a retrospective study in 335 patients. BMC Surg. 2017; 17:6.
Article60. Myles PS, Bellomo R, Corcoran T, Forbes A, Peyton P, Story D, et al. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med. 2018; 378:2263–74.
Article61. Myles PS, McIlroy DR, Bellomo R, Wallace S. Importance of intraoperative oliguria during major abdominal surgery: findings of the restrictive versus liberal fluid therapy in major abdominal surgery trial. Br J Anaesth. 2019; 122:726–33.
Article62. Jalil BA, Cavallazzi R. Predicting fluid responsiveness: a review of literature and a guide for the clinician. Am J Emerg Med. 2018; 36:2093–102.
Article63. Holte K, Klarskov B, Christensen DS, Lund C, Nielsen KG, Bie P, et al. Liberal versus restrictive fluid administration to improve recovery after laparoscopic cholecystectomy: a randomized, double-blind study. Ann Surg. 2004; 240:892–9.64. Holte K, Kristensen BB, Valentiner L, Foss NB, Husted H, Kehlet H. Liberal versus restrictive fluid management in knee arthroplasty: a randomized, double-blind study. Anesth Analg. 2007; 105:465–74.
Article65. Wu Y, Yang R, Xu J, Rusidanmu A, Zhang X, Hu J. Effects of intraoperative fluid management on postoperative outcomes after lobectomy. Ann Thorac Surg. 2019; 107:1663–9.
Article66. Malbrain ML, van Regenmortel N, Saugel B, De Tavernier B, van Gaal PJ, Joannes-Boyau O, et al. Principles of fluid management and stewardship in septic shock: it is time to consider the four D’s and the four phases of fluid therapy. Ann Intensive Care. 2018; 8:66.
Article67. Casey JD, Brown RM, Semler MW. Resuscitation fluids. Curr Opin Crit Care. 2018; 24:512–8.
Article68. Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001; 345:1368–77.
Article69. Marik PE. Iatrogenic salt water drowning and the hazards of a high central venous pressure. Ann Intensive Care. 2014; 4:21.
Article70. Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015; 372:1301–11.
Article71. ARISE Investigators, ANZICS Clinical Trials Group, Peake SL, Delaney A, Bailey M, Bellomo R, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014; 371:1496–506.
Article72. ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, et al. A randomized trial of protocolbased care for early septic shock. N Engl J Med. 2014; 370:1683–93.
Article73. Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med. 2018; 44:925–8.
Article74. Corl KA, Prodromou M, Merchant RC, Gareen I, Marks S, Banerjee D, et al. The restrictive IV fluid trial in severe sepsis and septic shock (RIFTS): a randomized pilot study. Crit Care Med. 2019; 47:951–9.75. Brown RM, Semler MW. Fluid management in sepsis. J Intensive Care Med. 2019; 34:364–73.
Article76. Li Y, He R, Ying X, Hahn RG. Ringer’s lactate, but not hydroxyethyl starch, prolongs the food intolerance time after major abdominal surgery; an open-labelled clinical trial. BMC Anesthesiol. 2015; 15:72.
Article77. Hahn RG, Bergek C, Gebäck T, Zdolsek J. Interactions between the volume effects of hydroxyethyl starch 130/0.4 and Ringer’s acetate. Crit Care. 2013; 17:R104.
Article78. Hippensteel JA, Uchimido R, Tyler PD, Burke RC, Han X, Zhang F, et al. Intravenous fluid resuscitation is associated with septic endothelial glycocalyx degradation. Crit Care. 2019; 23:259.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Influence of Daily Fluid Balance prior to Continuous Renal Replacement Therapy on Outcomes in Critically Ill Patients
- ED OVERCROWDING AND SPECIAL UNIT FOR OBSERVATION
- Fluid management in patients undergoing neurosurgery
- Characteristics of Critically Ill COVID-19 Patients in Busan, Republic of Korea
- Glycemic Targets for the Critically Ill Patient