J Korean Med Sci.
2016 Aug;31(8):1337-1344. 10.3346/jkms.2016.31.8.1337.
Influence of Daily Fluid Balance prior to Continuous Renal Replacement Therapy on Outcomes in Critically Ill Patients
- Affiliations
-
- 1Department of Internal Medicine, Chung-Ang University Hospital, Seoul, Korea. junghoshin1982@gmail.com
- KMID: 2373765
- DOI: http://doi.org/10.3346/jkms.2016.31.8.1337
Abstract
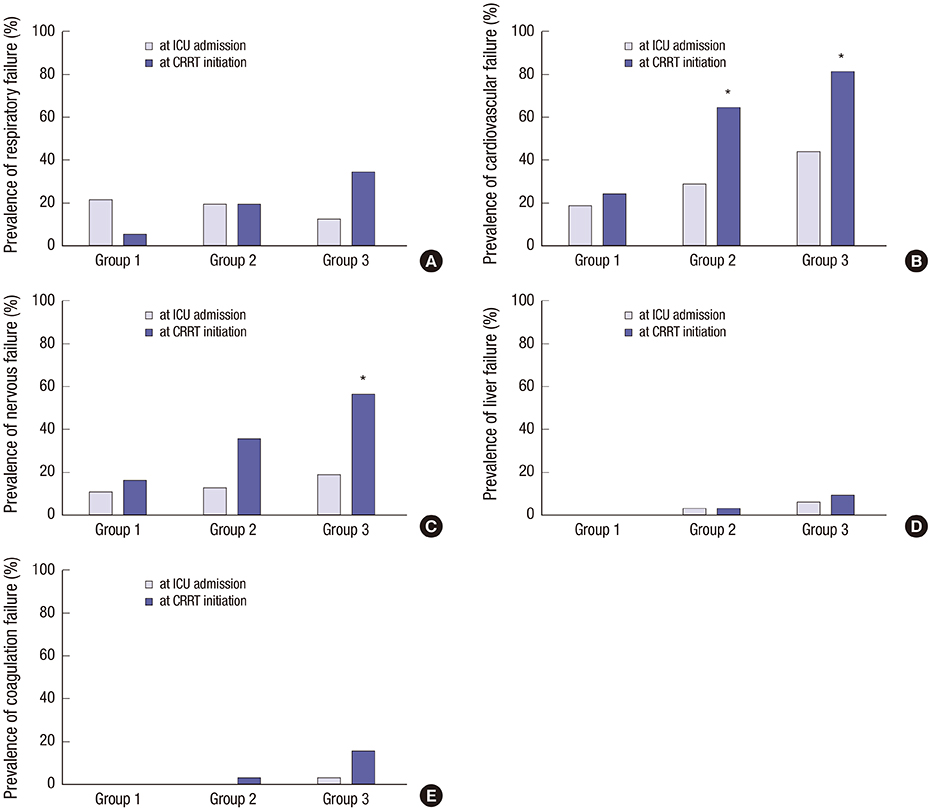
- Positive fluid balance is a risk factor for mortality in critically ill patients, especially those requiring continuous renal replacement therapy (CRRT). However, the association between daily fluid balance and various organ impairments remains unclear. This study investigated the impacts of daily fluid balance prior to CRRT on organ dysfunction, as well as mortality in critically ill patients. We identified daily fluid balance between intensive care unit (ICU) admission and CRRT initiation. According to daily fluid balance, the time to CRRT initiation and the rate of organ failure based on the sequential organ failure assessment (SOFA) score were assessed. We recruited 100 patients who experienced CRRT for acute kidney injury. CRRT was initiated within 2 [0, 4] days. The time to CRRT initiation was shortened in proportion to daily fluid balance, even after the adjustment for the renal SOFA score at ICU admission (HR 1.14, P = 0.007). Based on the SOFA score, positive daily fluid balance was associated with respiratory, cardiovascular, nervous, and coagulation failure, independent of each initial SOFA score at ICU admission (HR 1.36, 1.26, 1.24 and 2.26, all P < 0.05). Ultimately, we found that positive fluid balance was related with an increase in the rate of 28-day mortality (HR 1.14, P = 0.012). Positive daily fluid balance may accelerate the requirement for CRRT, moreover, it can be associated with an increased risk of multiple organ failure in critically ill patients.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
The Complexities of Intravenous Fluid Research: Questions of Scale, Volume, and Accumulation
Neil J Glassford, Rinaldo Bellomo
Korean J Crit Care Med. 2016;31(4):276-299. doi: 10.4266/kjccm.2016.00934.Utility of Volume Assessment Using Bioelectrical Impedance Analysis in Critically Ill Patients Receiving Continuous Renal Replacement Therapy: A Prospective Observational Study
Ki Hyun Park, Jung-ho Shin, Jin Ho Hwang, Su Hyun Kim
Korean J Crit Care Med. 2017;32(3):256-264. doi: 10.4266/kjccm.2017.00136.
Reference
-
1. Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M; Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001; 345:1368–1377.2. Prowle JR, Echeverri JE, Ligabo EV, Ronco C, Bellomo R. Fluid balance and acute kidney injury. Nat Rev Nephrol. 2010; 6:107–115.3. Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL; Program to Improve Care in Acute Renal Disease (PICARD) Study Group. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009; 76:422–427.4. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006; 354:2564–2575.5. Upadya A, Tilluckdharry L, Muralidharan V, Amoateng-Adjepong Y, Manthous CA. Fluid balance and weaning outcomes. Intensive Care Med. 2005; 31:1643–1647.6. Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005; 294:813–818.7. Piccinni P, Cruz DN, Gramaticopolo S, Garzotto F, Dal Santo M, Aneloni G, Rocco M, Alessandri E, Giunta F, Michetti V, et al. Prospective multicenter study on epidemiology of acute kidney injury in the ICU: a critical care nephrology Italian collaborative effort (NEFROINT). Minerva Anestesiol. 2011; 77:1072–1083.8. Bagshaw SM, Laupland KB, Doig CJ, Mortis G, Fick GH, Mucenski M, Godinez-Luna T, Svenson LW, Rosenal T. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: a population-based study. Crit Care. 2005; 9:R700–9.9. Kellum JA, Cerda J, Kaplan LJ, Nadim MK, Palevsky PM. Fluids for prevention and management of acute kidney injury. Int J Artif Organs. 2008; 31:96–110.10. Leblanc M, Kellum JA, Gibney RT, Lieberthal W, Tumlin J, Mehta R. Risk factors for acute renal failure: inherent and modifiable risks. Curr Opin Crit Care. 2005; 11:533–536.11. Vaara ST, Pettilä V, Reinikainen M, Kaukonen KM; Finnish Intensive Care Consortium. Population-based incidence, mortality and quality of life in critically ill patients treated with renal replacement therapy: a nationwide retrospective cohort study in Finnish intensive care units. Crit Care. 2012; 16:R13.12. Prescott GJ, Metcalfe W, Baharani J, Khan IH, Simpson K, Smith WC, MacLeod AM. A prospective national study of acute renal failure treated with RRT: incidence, aetiology and outcomes. Nephrol Dial Transplant. 2007; 22:2513–2519.13. Teixeira C, Garzotto F, Piccinni P, Brienza N, Iannuzzi M, Gramaticopolo S, Forfori F, Pelaia P, Rocco M, Ronco C, et al. Fluid balance and urine volume are independent predictors of mortality in acute kidney injury. Crit Care. 2013; 17:R14.14. Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL; Sepsis Occurrence in Acutely Ill Patients (SOAP) Investigators. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008; 12:R74.15. RENAL Replacement Therapy Study Investigators. An observational study fluid balance and patient outcomes in the randomized evaluation of normal vs. augmented level of replacement therapy trial. Crit Care Med. 2012; 40:1753–1760.16. Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998; 26:1793–1800.17. Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011; 39:259–265.18. Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006; 34:344–353.19. Micek ST, McEvoy C, McKenzie M, Hampton N, Doherty JA, Kollef MH. Fluid balance and cardiac function in septic shock as predictors of hospital mortality. Crit Care. 2013; 17:R246.20. Silversides JA, Pinto R, Kuint R, Wald R, Hladunewich MA, Lapinsky SE, Adhikari NK. Fluid balance, intradialytic hypotension, and outcomes in critically ill patients undergoing renal replacement therapy: a cohort study. Crit Care. 2014; 18:624.21. Vaara ST, Korhonen AM, Kaukonen KM, Nisula S, Inkinen O, Hoppu S, Laurila JJ, Mildh L, Reinikainen M, Lund V, et al. Fluid overload is associated with an increased risk for 90-day mortality in critically ill patients with renal replacement therapy: data from the prospective FINNAKI study. Crit Care. 2012; 16:R197.22. Van Biesen W, Yegenaga I, Vanholder R, Verbeke F, Hoste E, Colardyn F, Lameire N. Relationship between fluid status and its management on acute renal failure (ARF) in intensive care unit (ICU) patients with sepsis: a prospective analysis. J Nephrol. 2005; 18:54–60.23. de Oliveira FS, Freitas FG, Ferreira EM, de Castro I, Bafi AT, de Azevedo LC, Machado FR. Positive fluid balance as a prognostic factor for mortality and acute kidney injury in severe sepsis and septic shock. J Crit Care. 2015; 30:97–101.24. Burnett JC Jr, Knox FG. Renal interstitial pressure and sodium excretion during renal vein constriction. Am J Physiol. 1980; 238:F279–82.25. Shibagaki Y, Tai C, Nayak A, Wahba I. Intra-abdominal hypertension is an under-appreciated cause of acute renal failure. Nephrol Dial Transplant. 2006; 21:3567–3570.26. Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009; 53:582–588.27. Legrand M, Dupuis C, Simon C, Gayat E, Mateo J, Lukaszewicz AC, Payen D. Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: a retrospective observational study. Crit Care. 2013; 17:R278.28. Schrier RW. Fluid administration in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2010; 5:733–739.29. Epstein CD, Peerless JR. Weaning readiness and fluid balance in older critically ill surgical patients. Am J Crit Care. 2006; 15:54–64.30. Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004; 351:159–169.31. Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, Duchesne J, Bjorck M, Leppaniemi A, Ejike JC, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013; 39:1190–1206.32. Kramer AA, Wijdicks EF, Snavely VL, Dunivan JR, Naranjo LL, Bible S, Rohs T, Dickess SM. A multicenter prospective study of interobserver agreement using the full outline of unresponsiveness score coma scale in the intensive care unit. Crit Care Med. 2012; 40:2671–2676.33. Schreiber MA. Coagulopathy in the trauma patient. Curr Opin Crit Care. 2005; 11:590–597.34. Firth JD, Raine AE, Ledingham JG. Raised venous pressure: a direct cause of renal sodium retention in oedema? Lancet. 1988; 1:1033–1035.35. Diebel LN, Wilson RF, Dulchavsky SA, Saxe J. Effect of increased intra-abdominal pressure on hepatic arterial, portal venous, and hepatic microcirculatory blood flow. J Trauma. 1992; 33:279–282.36. Sheth RA, Arellano RS, Uppot RN, Samir AE, Goyal L, Zhu AX, Gervais DA, Mahmood U. Prospective trial with optical molecular imaging for percutaneous interventions in focal hepatic lesions. Radiology. 2015; 274:917–926.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Experiences of Pump-driven Continuous Venovenous Hemofiltration Therapy in Pediatric Patients
- Utility of Volume Assessment Using Bioelectrical Impedance Analysis in Critically Ill Patients Receiving Continuous Renal Replacement Therapy: A Prospective Observational Study
- Continuous Renal Replacement Therapy in Children
- Regional Citrate Anticoagulation in Continuous Venovenous Hemodiafiltration: Report of Two Cases
- Assessment of body fluid in critically ill patients with acute kidney injury requiring continuous renal replacement therapy