Anesth Pain Med.
2021 Jul;16(3):215-224. 10.17085/apm.21072.
Fluid management in patients undergoing neurosurgery
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Daegu Catholic University School of Medicine, Daegu, Korea
- KMID: 2519050
- DOI: http://doi.org/10.17085/apm.21072
Abstract
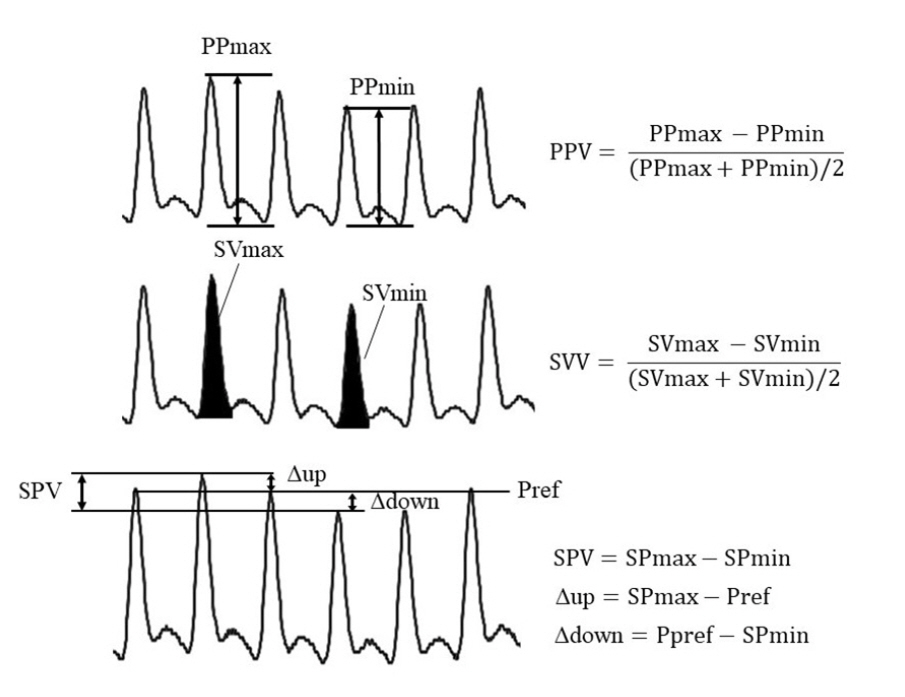
- Fluid management is an important component of perioperative care for patients undergoing neurosurgery. The primary goal of fluid management in neurosurgery is the maintenance of normovolemia and prevention of serum osmolarity reduction. To maintain normovolemia, it is important to administer fluids in appropriate amounts following appropriate methods, and to prevent a decrease in serum osmolarity, the choice of fluid is essential. There is considerable debate about the choice and optimal amounts of fluids administered in the perioperative period. However, there is little high-quality clinical research on fluid therapy for patients undergoing neurosurgery. This review will discuss the choice and optimal amounts of fluids in neurosurgical patients based on the literature, recent issues, and perioperative fluid management practices.
Figure
Reference
-
1. Wrzosek A, Jakowicka-Wordliczek J, Zajaczkowska R, Serednicki WT, Jankowski M, Bala MM, et al. Perioperative restrictive versus goal-directed fluid therapy for adults undergoing major non-cardiac surgery. Cochrane Database Syst Rev. 2019; 12:CD012767.2. Brandstrup B, Tønnesen H, Beier-Holgersen R, Hjortsø E, Ørding H, Lindorff-Larsen K, et al. Danish Study Group on Perioperative Fluid Therapy. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003; 238:641–8.3. Myles PS, Bellomo R, Corcoran T, Forbes A, Peyton P, Story D, et al. Australian and New Zealand College of Anaesthetists Clinical Trials Network and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med. 2018; 378:2263–74.4. Tommasino C, Picozzi V. Volume and electrolyte management. Best Pract Res Clin Anaesthesiol. 2007; 21:497–516.5. Drummond JC, Patel PM, Cole DJ, Kelly PJ. The effect of the reduction of colloid oncotic pressure, with and without reduction of osmolality, on post-traumatic cerebral edema. Anesthesiology. 1998; 88:993–1002.6. Reinhart K, Perner A, Sprung CL, Jaeschke R, Schortgen F, Johan Groeneveld AB, et al. European Society of Intensive Care Medicine. Consensus statement of the ESICM task force on colloid volume therapy in critically ill patients. Intensive Care Med. 2012; 38:368–83.7. Cooper DJ, Myburgh J, Heritier S, Finfer S, Bellomo R, Billot L, et al. SAFE-TBI Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group. Albumin resuscitation for traumatic brain injury: is intracranial hypertension the cause of increased mortality? J Neurotrauma. 2013; 30:512–8.8. Quilley CP, Lin YS, McGiff JC. Chloride anion concentration as a determinant of renal vascular responsiveness to vasoconstrictor agents. Br J Pharmacola. 1993; 108:106–10.9. Shaw AD, Bagshaw SM, Goldstein SL, Scherer LA, Duan M, Schermer CR, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma-Lyte. Ann Surg. 2012; 255:821–9.10. McCluskey SA, Karkouti K, Wijeysundera D, Minkovich L, Tait G, Beattie WS. Hyperchloremia after noncardiac surgery is independently associated with increased morbidity and mortality: a propensity-matched cohort study. Anesth Analg. 2013; 117:412–21.11. Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, et al. SMART Investigators and the Pragmatic Critical Care Research Group. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018; 378:829–39.12. Young P, Bailey M, Beasley R, Henderson S, Mackle D, McArthur C, et al. SPLIT Investigators; ANZICS CTG. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. JAMA. 2015; 314:1701–10.13. Self WH, Semler MW, Wanderer JP, Wang L, Byrne DW, Collins SP, et al. SALT-ED Investigators. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018; 378:819–28.14. Hassan MH, Hassan WMNW, Zaini RHM, Shukeri WFWM, Abidin HZ, Eu CS. Balanced fluid versus saline-based fluid in post-operative severe traumatic brain injury patients: acid-base and electrolytes assessment. Malays J Med Sci. 2017; 24:83–93.15. Odor PM, Bampoe S, Dushianthan A, Bennett-Guerrero E, Cro S, Gan TJ, et al. Perioperative administration of buffered versus non-buffered crystalloid intravenous fluid to improve outcomes following adult surgical procedures: a Cochrane systematic review. Perioper Med (Lond). 2018; 7:27.16. Dąbrowski W, Woodcock T, Rzecki Z, Malbrain MLNG. The use of crystalloids in traumatic brain injury. Anaesthesiol Intensive Ther. 2018; 50:150–9.17. Hammond NE, Taylor C, Finfer S, Machado FR, An Y, Billot L, et al. Fluid-TRIPS and Fluidos Investigators; George Institute for Global Health, The ANZICS Clinical Trials Group, BRICNet, and the REVA research Network. Patterns of intravenous fluid resuscitation use in adult intensive care patients between 2007 and 2014: an international cross-sectional study. PLoS One. 2017; 12:e0176292.18. Hafizah M, Liu CY, Ooi JS. Normal saline versus balanced-salt solution as intravenous fluid therapy during neurosurgery: effects on acid-base balance and electrolytes. J Neurosurg Sci. 2017; 61:263–70.19. Hladky SB, Barrand MA. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS. 2014; 11:26.20. Rowell SE, Fair KA, Barbosa RR, Watters JM, Bulger EM, Holcomb JB, et al. The impact of pre-hospital administration of lactated Ringer's solution versus normal saline in patients with traumatic brain injury. J Neurotrauma. 2016; 33:1054–9.21. Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017; 80:6–15.22. Roquilly A, Loutrel O, Cinotti R, Rosenczweig E, Flet L, Mahe PJ, et al. Balanced versus chloride-rich solutions for fluid resuscitation in brain-injured patients: a randomised double-blind pilot study. Crit Care. 2013; 17:R77.23. Exo JL, Shellington DK, Bayir H, Vagni VA, Janesco-Feldman K, Ma L, et al. Resuscitation of traumatic brain injury and hemorrhagic shock with polynitroxylated albumin, hextend, hypertonic saline, and lactated Ringer's: effects on acute hemodynamics, survival, and neuronal death in mice. J Neurotrauma. 2009; 26:2403–8.24. Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. German Competence Network Sepsis (SepNet). Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008; 358:125–39.25. Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Åneman A, et al. 6S Trial Group; Scandinavian Critical Care Trials Group. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med. 2012; 367:124–34.26. Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, et al. CHEST Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012; 367:1901–11.27. Guidet B, Martinet O, Boulain T, Philippart F, Poussel JF, Maizel J, et al. Assessment of hemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: the CRYSTMAS study. Crit Care. 2012; 16:R94.28. Annane D, Siami S, Jaber S, Martin C, Elatrous S, Declère AD, et al. CRISTAL Investigators. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA. 2013; 310:1809–17.29. Zarychanski R, Abou-Setta AM, Turgeon AF, Houston BL, McIntyre L, Marshall JC, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA. 2013; 309:678–88.30. Lewis SR, Pritchard MW, Evans DJ, Butler AR, Alderson P, Smith AF, et al. Colloids versus crystalloids for fluid resuscitation in critically ill people. Cochrane Database Syst Rev. 2018; 8:CD000567.31. Gerhartl A, Hahn K, Neuhoff A, Friedl HP, Förster CY, Wunder C, et al. Hydroxyethylstarch (130/0.4) tightens the blood-brain barrier in vitro. Brain Res. 2020; 1727:146560.32. Schick MA, Burek M, Förster CY, Nagai M, Wunder C, Neuhaus W. Hydroxyethylstarch revisited for acute brain injury treatment. Neural Regen Res. 2021; 16:1372–6.33. Chi OZ, Lu X, Wei HM, Williams JA, Weiss HR. Hydroxyethyl starch solution attenuates blood-brain barrier disruption caused by intracarotid injection of hyperosmolar mannitol in rats. Anesth Analg. 1996; 83:336–41.34. Woessner R, Grauer MT, Dieterich HJ, Bepperling F, Baus D, Kahles T, et al. Influence of a long-term, high-dose volume therapy with 6% hydroxyethyl starch 130/0.4 or crystalloid solution on hemodynamics, rheology and hemostasis in patients with acute ischemic stroke. Results of a randomized, placebo-controlled, double-blind study. Pathophysiol Haemost Thromb. 2003; 33:121–6.35. Rudolf J; HES in Acute Stroke Study Group. Hydroxyethyl starch for hypervolemic hemodilution in patients with acute ischemic stroke: a randomized, placebo-controlled phase II safety study. Cerebrovasc Dis. 2002; 14:33–41.36. Mutoh T, Kazumata K, Ishikawa T, Terasaka S. Performance of bedside transpulmonary thermodilution monitoring for goal-directed hemodynamic management after subarachnoid hemorrhage. Stroke. 2009; 40:2368–74.37. Bercker S, Winkelmann T, Busch T, Laudi S, Lindner D, Meixensberger J. Hydroxyethyl starch for volume expansion after subarachnoid haemorrhage and renal function: results of a retrospective analysis. PLoS One. 2018; 13:e0192832.38. Kunze E, Stetter C, Willner N, Koehler S, Kilgenstein C, Ernestus RI, et al. Effects of fluid treatment with hydroxyethyl starch on renal function in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2016; 28:187–94.39. Huh PW, Belayev L, Zhao W, Busto R, Saul I, Ginsberg MD. The effect of high-dose albumin therapy on local cerebral perfusion after transient focal cerebral ischemia in rats. Brain Res. 1998; 804:105–13.40. Belayev L, Saul I, Huh PW, Finotti N, Zhao W, Busto R, et al. Neuroprotective effect of high-dose albumin therapy against global ischemic brain injury in rats. Brain Res. 1999; 845:107–11.41. Belayev L, Liu Y, Zhao W, Busto R, Ginsberg MD. Human albumin therapy of acute ischemic stroke: marked neuroprotective efficacy at moderate doses and with a broad therapeutic window. Stroke. 2001; 32:553–60.42. Suarez JI, Shannon L, Zaidat OO, Suri MF, Singh G, Lynch G, et al. Effect of human albumin administration on clinical outcome and hospital cost in patients with subarachnoid hemorrhage. J Neurosurg. 2004; 100:585–90.43. Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004; 350:2247–56.44. SAFE Study Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group; Australian Red Cross Blood Service; George Institute for International Health, Myburgh J, Cooper DJ, Finfer S, Bellomo R, Norton R, et al. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007; 357:874–84.45. Huijben JA, Volovici V, Cnossen MC, Haitsma IK, Stocchetti N, Maas AIR, et al. CENTER-TBI investigators and participants. Variation in general supportive and preventive intensive care management of traumatic brain injury: a survey in 66 neurotrauma centers participating in the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) study. Crit Care. 2018; 22:90.46. Van Aken HK, Kampmeier TG, Ertmer C, Westphal M. Fluid resuscitation in patients with traumatic brain injury: what is a SAFE approach? Curr Opin Anaesthesiol. 2012; 25:563–5.47. Wang L, Li M, Xie Y, Xu L, Ye R, Liu X. Preclinical efficacy of human albumin in subarachnoid hemorrhage. Neuroscience. 2017; 344:255–64.48. Belayev L, Pinard E, Nallet H, Seylaz J, Liu Y, Riyamongkol P, et al. Albumin therapy of transient focal cerebral ischemia: in vivo analysis of dynamic microvascular responses. Stroke. 2002; 33:1077–84.49. Suarez JI, Martin RH, Calvillo E, Dillon C, Bershad EM, Macdonald RL, et al. ALISAH Investigators. The Albumin in Subarachnoid Hemorrhage (ALISAH) multicenter pilot clinical trial: safety and neurologic outcomes. Stroke. 2012; 43:683–90.50. Suarez JI, Martin RH, Calvillo E, Bershad EM, Venkatasubba Rao CP. Effect of human albumin on TCD vasospasm, DCI, and cerebral infarction in subarachnoid hemorrhage: the ALISAH study. Acta Neurochir Suppl. 2015; 120:287–90.51. Palesch YY, Hill MD, Ryckborst KJ, Tamariz D, Ginsberg MD. The ALIAS pilot trial: a dose-escalation and safety study of albumin therapy for acute ischemic stroke--II: neurologic outcome and efficacy analysis. Stroke. 2006; 37:2107–14.52. Ginsberg MD, Palesch YY, Martin RH, Hill MD, Moy CS, Waldman BD, et al. ALIAS Investigators. The albumin in acute stroke (ALIAS) multicenter clinical trial: safety analysis of part 1 and rationale and design of part 2. Stroke. 2011; 42:119–27.53. Ginsberg MD, Palesch YY, Hill MD, Martin RH, Moy CS, Barsan WG, et al. ALIAS and Neurological Emergencies Treatment Trials (NETT) Investigators. High-dose albumin treatment for acute ischaemic stroke (ALIAS) Part 2: a randomised, double-blind, phase 3, placebo-controlled trial. Lancet Neurol. 2013; 12:1049–58.54. Martin RH, Yeatts SD, Hill MD, Moy CS, Ginsberg MD, Palesch YY; ALIAS Parts 1 and 2 and NETT Investigators. ALIAS (albumin in acute ischemic stroke) trials: analysis of the combined data from parts 1 and 2. Stroke. 2016; 47:2355–9.55. Oddo M, Poole D, Helbok R, Meyfroidt G, Stocchetti N, Bouzat P, et al. Fluid therapy in neurointensive care patients: ESICM consensus and clinical practice recommendations. Intensive Care Med. 2018; 44:449–63.56. Shin CH, Long DR, McLean D, Grabitz SD, Ladha K, Timm FP, et al. Effects of intraoperative fluid management on postoperative outcomes: a hospital registry study. Ann Surg. 2018; 267:1084–92.57. Thacker JK, Mountford WK, Ernst FR, Krukas MR, Mythen MM. Perioperative fluid utilization variability and association with outcomes: considerations for enhanced recovery efforts in sample US surgical populations. Ann Surg. 2016; 263:502–10.58. Bundgaard-Nielsen M, Secher NH, Kehlet H. 'Liberal' vs. 'restrictive' perioperative fluid therapy--a critical assessment of the evidence. Acta Anaesthesiol Scand. 2009; 53:843–51.59. Benes J, Giglio M, Brienza N, Michard F. The effects of goal-directed fluid therapy based on dynamic parameters on post-surgical outcome: a meta-analysis of randomized controlled trials. Crit Care. 2014; 18:584.60. Corcoran T, Rhodes JE, Clarke S, Myles PS, Ho KM. Perioperative fluid management strategies in major surgery: a stratified meta-analysis. Anesth Analg. 2012; 114:640–51.61. Michard F, Mountford WK, Krukas MR, Ernst FR, Fogel SL. Potential return on investment for implementation of perioperative goal-directed fluid therapy in major surgery: a nationwide database study. Perioper Med (Lond). 2015; 4:11.62. Colantonio L, Claroni C, Fabrizi L, Marcelli ME, Sofra M, Giannarelli D, et al. A randomized trial of goal directed vs. standard fluid therapy in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. J Gastrointest Surg. 2015; 19:722–9.63. Kissoon NR, Mandrekar JN, Fugate JE, Lanzino G, Wijdicks EF, Rabinstein AA. Positive fluid balance is associated with poor outcomes in subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2015; 24:2245–51.64. Martini RP, Deem S, Brown M, Souter MJ, Yanez ND, Daniel S, et al. The association between fluid balance and outcomes after subarachnoid hemorrhage. Neurocrit Care. 2012; 17:191–8.65. Li J, Ji FH, Yang JP. Evaluation of stroke volume variation obtained by the FloTrac™/Vigileo™ system to guide preoperative fluid therapy in patients undergoing brain surgery. J Int Med Res. 2012; 40:1175–81.66. Berkenstadt H, Margalit N, Hadani M, Friedman Z, Segal E, Villa Y, et al. Stroke volume variation as a predictor of fluid responsiveness in patients undergoing brain surgery. Anesth Analg. 2001; 92:984–9.67. Wu CY, Lin YS, Tseng HM, Cheng HL, Lee TS, Lin PL, et al. Comparison of two stroke volume variation-based goal-directed fluid therapies for supratentorial brain tumour resection: a randomized controlled trial. Br J Anaesth. 2017; 119:934–42.68. Luo J, Xue J, Liu J, Liu B, Liu L, Chen G. Goal-directed fluid restriction during brain surgery: a prospective randomized controlled trial. Ann Intensive Care. 2017; 7:16.69. Sundaram SC, Salins SR, Kumar AN, Korula G. Intra-operative fluid management in adult neurosurgical patients undergoing intracranial tumour surgery: randomised control trial comparing pulse pressure variance (PPV) and central venous pressure (CVP). J Clin Diagn Res. 2016; 10:UC01–5.70. Hasanin A, Zanata T, Osman S, Abdelwahab Y, Samer R, Mahmoud M, et al. Pulse pressure variation-guided fluid therapy during supratentorial brain tumour excision: a randomized controlled trial. Open Access Maced J Med Sci. 2019; 7:2474–9.71. Bapteste L, Carrillon R, Javelier S, Guyotat J, Desgranges FP, Lehot JJ, et al. Pulse pressure variations and plethysmographic variability index measured at ear are able to predict fluid responsiveness in the sitting position for neurosurgery. J Neurosurg Anesthesiol. 2020; 32:263–7.72. Byon HJ, Lim CW, Lee JH, Park YH, Kim HS, Kim CS, et al. Prediction of fluid responsiveness in mechanically ventilated children undergoing neurosurgery. Br J Anaesth. 2013; 110:586–91.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anesthetic management of the traumatic brain injury patients undergoing non-neurosurgery
- Traumatic Cerebrospinal Fluid Leak: Diagnosis and Management
- Management of Cerebrospinal Fluid Rhinorrhea
- The Experience of Fluid Management in Hemodialysis Patients
- Risk Factors of Pressure Injury related to Surgery in Neurosurgery Patients