Investig Clin Urol.
2020 Jan;61(1):88-98. 10.4111/icu.2020.61.1.88.
Establishment of NOAEL for intracavernous injections of human bone marrow-derived mesenchymal stem cells in rats
- Affiliations
-
- 1Department of Urology, Hallym University Dongtan Sacred Heart Hospital, Hwaseong, Korea.
- 2Asan Institute for Life Science, Asan Medical Center, Seoul, Korea.
- 3Department of Urology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. cskim@amc.seoul.kr
- 4NKMAX Co., Ltd., Seongnam, Korea.
- 5Department of Urology, Kangwon National University School of Medicine, Chuncheon, Korea.
- 6Pharmicell Co., Ltd., Seongnam, Korea.
- 7Department of Pharmaceutical Engineering, College of Medical Sciences, Soon Chun Hyang University, Asan, Korea.
- KMID: 2466797
- DOI: http://doi.org/10.4111/icu.2020.61.1.88
Abstract
- PURPOSE
To assess the possible negative health effects of human bone marrow-derived mesenchymal stem cells (hBMSCs) on fertility and early embryonic development following intracavernous injections in rats.
MATERIALS AND METHODS
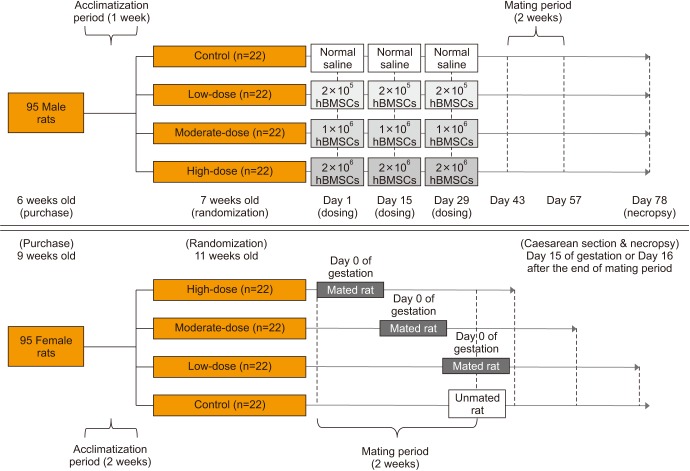
A total of 88 Crl:CD(SD) male and female rats were equally divided into 4 groups in a random manner: control group (normal saline), low-dose group (2×10ⵠhBMSCs), moderate-dose group (1×10ⶠhBMSCs), and high-dose group (2×10ⶠhBMSCs). hBMSCs or normal saline was injected into the penis of the rats 3 times at 2-week-intervals prior to mating. We compared each group with respect to parameters of reproduction and histopathology.
RESULTS
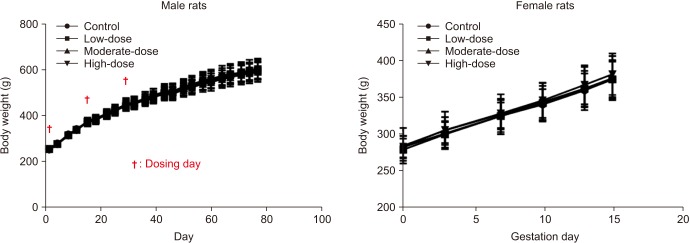
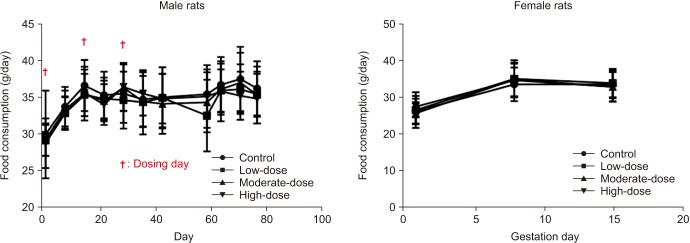
For male rats, various degrees of flushing and swelling were observed at the penile injection site in all the groups, although the severity increased in a dose-dependent manner in the hBMSC injection groups. There were no statistically significant differences in mean body weights and food consumption among all the groups of both sexes. There were no statistically significant differences in reproductive parameters among all the groups of both sexes. The absolute and relative organ weights did not significantly differ among the groups. At the time of necropsy, no remarkable findings were observed in gross examinations in all groups. On histopathological analysis, minimal mononuclear cell infiltration was observed in the right epididymis of each rat in the moderate- and high-dose groups.
CONCLUSIONS
The non-toxic amount of hBMSCs for male fertility and early embryogenesis in rats under the test conditions was determined to be 2×10ⶠcells/head.
MeSH Terms
Figure
Reference
-
1. McCabe MP, Sharlip ID, Atalla E, Balon R, Fisher AD, Laumann E, et al. Definitions of sexual dysfunctions in women and men: a consensus statement from the fourth International Consultation on Sexual Medicine 2015. J Sex Med. 2016; 13:135–143. PMID: 26953828.
Article2. Goldstein I, Chambers R, Tang W, Stecher V, Hassan T. Realworld observational results from a database of 48 million men in the United States: relationship of cardiovascular disease, diabetes mellitus and depression with age and erectile dysfunction. Int J Clin Pract. 2018; 72:e13078. PMID: 29569323.
Article3. Cellek S, Rodrigo J, Lobos E, Fernández P, Serrano J, Moncada S. Selective nitrergic neurodegeneration in diabetes mellitusa nitric oxide-dependent phenomenon. Br J Pharmacol. 1999; 128:1804–1812. PMID: 10588937.
Article4. Ozkara H, Alan C, Atukeren P, Uyaner I, Demirci C, Gümüştaş MK, et al. Changes of nitric oxide synthase-containing nerve fibers and parameters for oxidative stress after unilateral cavernous nerve resection or manuplation in rat penis. Chin J Physiol. 2006; 49:160–166. PMID: 16970248.5. Suetomi T, Kawai K, Hinotsu S, Joraku A, Oikawa T, Sekido N, et al. Negative impact of metabolic syndrome on the responsiveness to sildenafil in Japanese men. J Sex Med. 2008; 5:1443–1450. PMID: 18208503.
Article6. Soebadi MA, Moris L, Castiglione F, Weyne E, Albersen M. Advances in stem cell research for the treatment of male sexual dysfunctions. Curr Opin Urol. 2016; 26:129–139. PMID: 26759972.
Article7. Alwaal A, Hussein AA, Lin CS, Lue TF. Prospects of stem cell treatment in benign urological diseases. Korean J Urol. 2015; 56:257–265. PMID: 25874038.
Article8. You D, Jang MJ, Lee J, Jeong IG, Kim HS, Moon KH, et al. Periprostatic implantation of human bone marrow-derived mesenchymal stem cells potentiates recovery of erectile function by intracavernosal injection in a rat model of cavernous nerve injury. Urology. 2013; 81:104–110. PMID: 23122545.
Article9. You D, Jang MJ, Kim BH, Choi KR, Lee C, Song G, et al. Bone marrow-derived mesenchymal stromal cell therapy in a rat model of cavernous nerve injury: preclinical study for approval. Cytotherapy. 2016; 18:870–880. PMID: 27260208.
Article10. Bahk JY, Jung JH, Han H, Min SK, Lee YS. Treatment of diabetic impotence with umbilical cord blood stem cell intracavernosal transplant: preliminary report of 7 cases. Exp Clin Transplant. 2010; 8:150–160. PMID: 20565373.11. Yiou R, Hamidou L, Birebent B, Bitari D, Lecorvoisier P, Contremoulins I, et al. Safety of intracavernous bone marrow-mononuclear cells for postradical prostatectomy erectile dysfunction: an open dose-escalation pilot study. Eur Urol. 2016; 69:988–991. PMID: 26439886.
Article12. Haahr MK, Jensen CH, Toyserkani NM, Andersen DC, Damkier P, Sørensen JA, et al. Safety and potential effect of a single intracavernous injection of autologous adipose-derived regenerative cells in patients with erectile dysfunction following radical prostatectomy: an open-label phase I clinical trial. EBioMedicine. 2016; 5:204–210. PMID: 27077129.
Article13. Carter A, Robison LL, Francisco L, Smith D, Grant M, Baker KS, et al. Prevalence of conception and pregnancy outcomes after hematopoietic cell transplantation: report from the Bone Marrow Transplant Survivor Study. Bone Marrow Transplant. 2006; 37:1023–1029. PMID: 16604098.
Article14. Wagner AM, Beier K, Christen E, Holländer GA, Krenger W. Leydig cell injury as a consequence of an acute graft-versus-host reaction. Blood. 2005; 105:2988–2990. PMID: 15576479.
Article15. Rovó A, Aljurf M, Chiodi S, Spinelli S, Salooja N, Sucak G, et al. Late Effects Working Party of the EBMT. Ongoing graft-versus-host disease is a risk factor for azoospermia after allogeneic hematopoietic stem cell transplantation: a survey of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2013; 98:339–345. PMID: 22929982.
Article16. Rengasamy M, Gupta PK, Kolkundkar U, Singh G, Balasubramanian S, SundarRaj S, et al. Preclinical safety & toxicity evaluation of pooled, allogeneic human bone marrow-derived mesenchymal stromal cells. Indian J Med Res. 2016; 144:852–864. PMID: 28474622.17. Yimam M, Lee YC, Hyun EJ, Jia Q. Reproductive and developmental toxicity of orally administered botanical composition, UP446-part III: effects on fertility and early embryonic development to implantation in Sprague Dawley rats. Birth Defects Res B Dev Reprod Toxicol. 2015; 104:166–176. PMID: 26173630.
Article18. Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003; 31:890–896. PMID: 14550804.19. Grinnemo KH, Månsson A, Dellgren G, Klingberg D, Wardell E, Drvota V, et al. Xenoreactivity and engraftment of human mesenchymal stem cells transplanted into infarcted rat myocardium. J Thorac Cardiovasc Surg. 2004; 127:1293–1300. PMID: 15115985.
Article20. Auchincloss H Jr, Sachs DH. Xenogeneic transplantation. Annu Rev Immunol. 1998; 16:433–470. PMID: 9597137.
Article21. Liang X, Ding Y, Zhang Y, Tse HF, Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014; 23:1045–1059. PMID: 23676629.
Article22. ClinicalTrials.gov. Safety of autologous bone marrow derived mesenchymal stem cells in erectile dysfunction [Internet]. Bethesda: U.S. National Library of Medicine;2015. 1. 26. updated 2019 Jan 9. cited 2016 Jul 31. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02344849.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Safety and Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Sensorineural Hearing Loss Patients
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Stem Cells and Niemann Pick Disease
- Bone marrow-derived stem cells contribute to regeneration of the endometrium
- Neural Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells: Applicability for Inner Ear Therapy