J Nutr Health.
2019 Dec;52(6):515-528. 10.4163/jnh.2019.52.6.515.
Splenocyte-mediated immune enhancing activity of Sargassum horneri extracts
- Affiliations
-
- 1Division of Natural Product Research, Korea Prime Pharmacy CO., LTD., Jeonnam 58144, Korea.
- 2Department of Food and Nutrition, Chungnam National University, Daejeon 34134, Korea. kakim@cnu.ac.kr
- KMID: 2466666
- DOI: http://doi.org/10.4163/jnh.2019.52.6.515
Abstract
- PURPOSE
This study examined the immunological activity and optimized the mixture conditions of Sargassum horneri (S. horneri) extracts in vitro and in vivo models.
METHODS
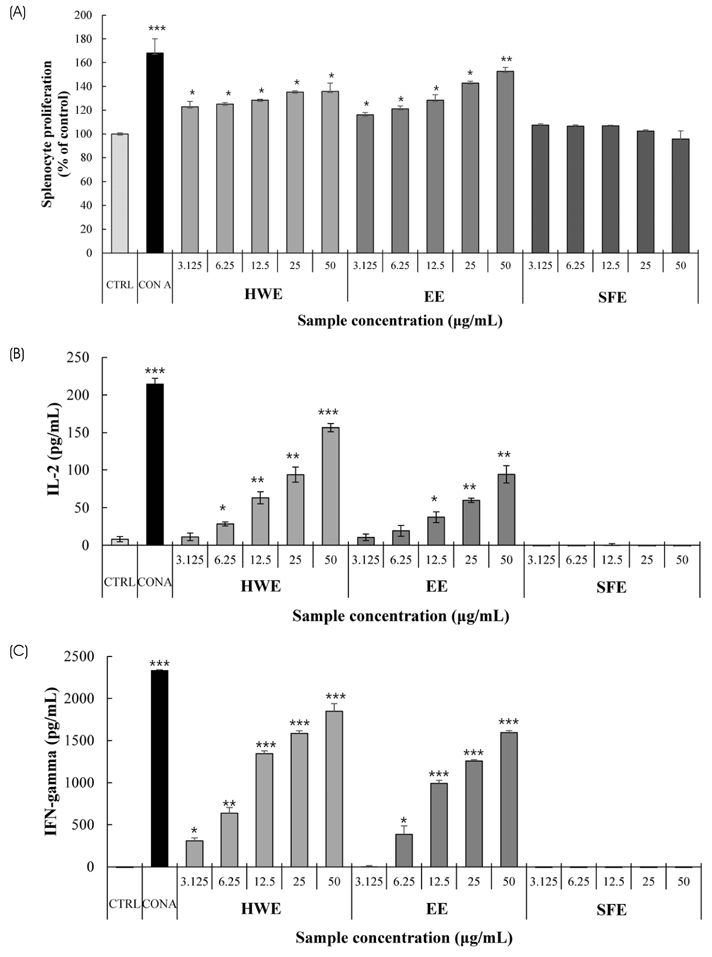
S. horneri was extracted using three different methods: hot water extraction (HWE), 50% ethanol extraction (EE), and supercritical fluid extraction (SFE). Splenocyte proliferation and cytokine production (Interleukin-2 and Interferon-γ) were measured using a WST-1 assay and enzyme-linked immunosorbent assay, respectively. The levels of nitric oxide and T cell activation production were measured using a Griess assay and flow cytometry, respectively. The natural killer (NK) cell activity was determined using an EZ-LDH kit.
RESULTS
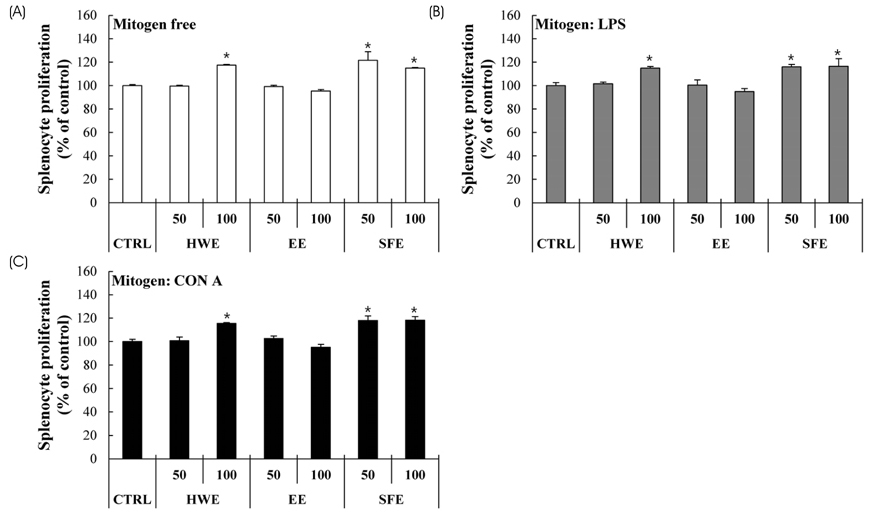
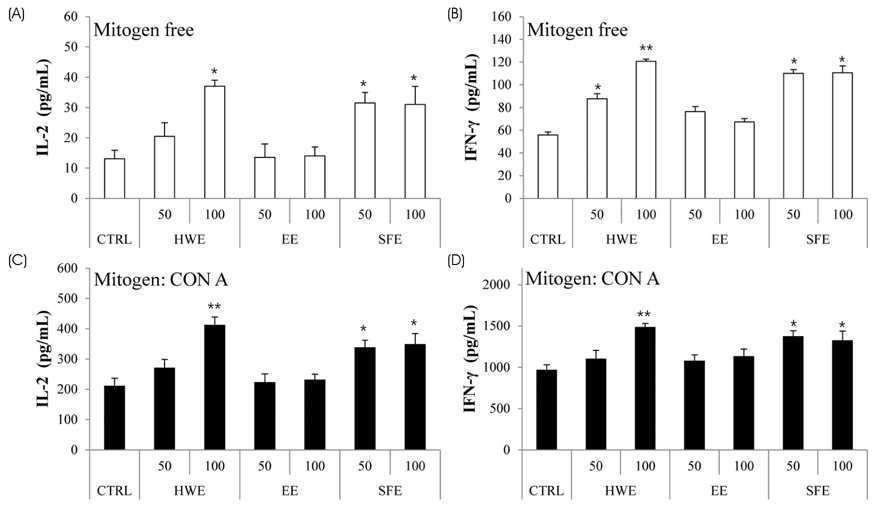
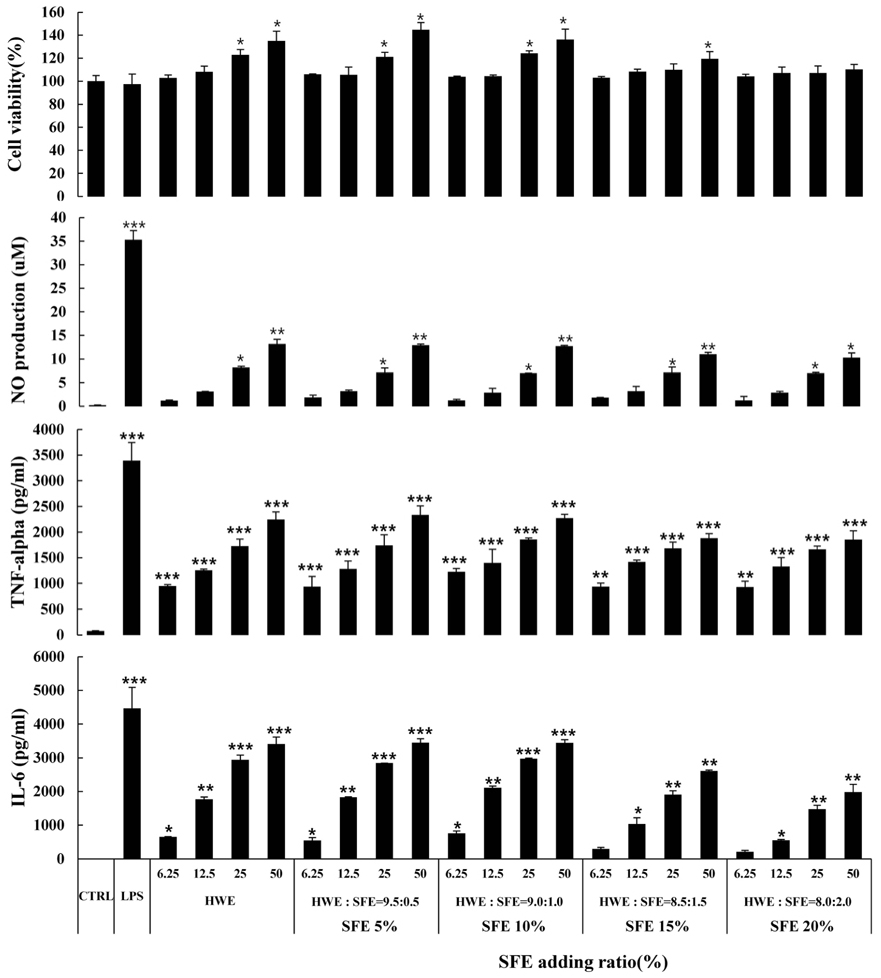
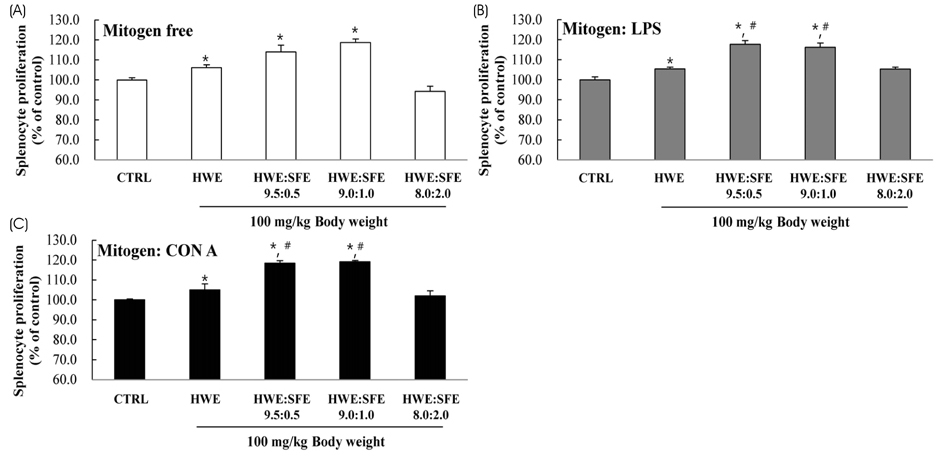
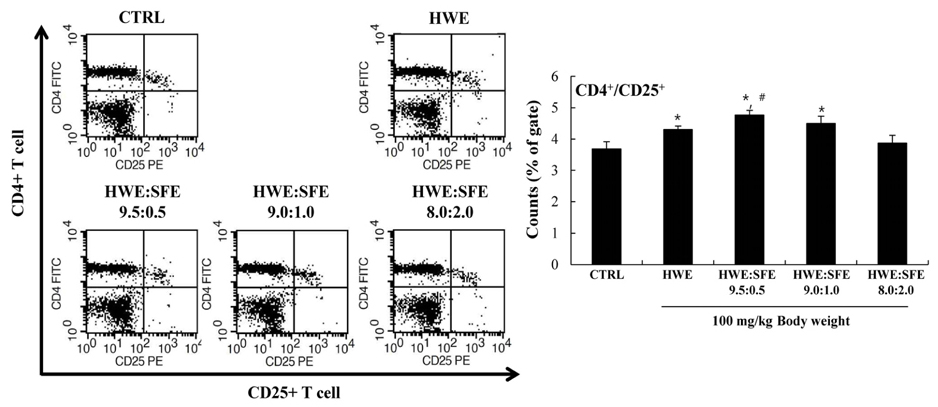
Among the three different types of extracts, HWE showed the highest levels of splenocyte proliferation and cytokine production in vitro. In the animal model, three different types of extracts were administrated for 14 days (once/day) at 50 and 100 mg/kg body weight. HWE and SFE showed a high level of splenocyte proliferation and cytokine production in the with and without mitogen-treated groups, whereas EE administration did not induce the splenocyte activation. When RAW264.7 macrophage cells were treated with different mixtures (HWE with 5, 10, 15, 20% of SFE) to determine the optimal mixture ratio of HWE and SFE, the levels of nitric oxide and cytokine production increased strongly in the HWE with 5% and 10% of SFE containing group. In the animal model, HWE with 5% and 10% of SFE mixture administration increased the levels of splenocyte proliferation, cytokine production, and activated CD4⺠cell population significantly, with the highest level observed in the HWE with 5% of SFE group. Moreover, the NK cell activity was increased significantly in the HWE with 5% of SFE mixture-treated group compared to the control group.
CONCLUSION
The optimal mixture condition of S. horneri with immune-enhancing activity is the HWE with 5% of SFE mixture. These results confirmed that the extracts of S. horneri and its mixtures are potential candidate materials for immune enhancement.
Keyword
MeSH Terms
Figure
Reference
-
1. Tomasi TB Jr, Tan EM, Solomon A, Prendergast RA. Characteristics of an immune system common to certain external secretions. J Exp Med. 1965; 121(1):101–124.
Article2. Byun EH. Immunomodulatory activities of crude polysaccharide fraction separated from Perilla frutescens Britton var. acuta Kudo. Korean J Food Sci Technol. 2017; 49(5):559–566.3. Cho EJ, Lee JH, Sung NY, Byun EH. Anti-inflammatory effects of Annona muricata leaf ethanol extracts. J Korean Soc Food Sci Nutr. 2017; 46(6):681–687.4. Kim YE, Lee JH, Sung NY, Ahn DH, Byun EH. A comparative study of the immuno-modulatory activities of ethanol extracts and crude polysaccharide fractions from Annona muricata L. Korean J Food Sci Technol. 2017; 49(4):453–458.5. Klimp AH, de Vries EG, Scherphof GL, Daemen T. A potential role of macrophage activation in the treatment of cancer. Crit Rev Oncol Hematol. 2002; 44(2):143–161.
Article6. Schepetkin IA, Quinn MT. Botanical polysaccharides: macrophage immunomodulation and therapeutic potential. Int Immunopharmacol. 2006; 6(3):317–333.
Article7. Sung NY, Park WY, Kim YE, Cho EJ, Song H, Jun HK, et al. Increase in antioxidant components and reduction of off-flavors on radish leaf extracts by extrusion process. J Korean Soc Food Sci Nutr. 2016; 45(12):1769–1775.
Article8. Kim JS, Oh CH, Jeon H, Lee KS, Ma SY. Immuno-regulatory property of fruit-extracts of Cornus kousa Burg. Korean J Med Crop Sci. 2002; 10(5):327–332.9. Lee JY, Hwang WI, Lim ST. Effect of Platycodon grandiflorum DC extract on the growth of cancer cell lines. Korean J Food Sci Technol. 1998; 30(1):13–21.10. Byrd JC, Park JH, Schaffer BS, Garmroudi F, MacDonald RG. Dimerization of the insulin-like growth factor II/mannose 6-phosphate receptor. J Biol Chem. 2000; 275(25):18647–18656.
Article11. Brown ES, Allsopp PJ, Magee PJ, Gill CI, Nitecki S, Strain CR, et al. Seaweed and human health. Nutr Rev. 2014; 72(3):205–216.
Article12. Kim NG. Effects of temperature, photon irradiance, and photoperiod on the growth of embryos of Sargassum horneri in laboratory culture. Korean J Fish Aquat Sci. 2015; 48(1):76–81.
Article13. Liu L, Heinrich M, Myers S, Dworjanyn SA. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in Traditional Chinese Medicine: a phytochemical and pharmacological review. J Ethnopharmacol. 2012; 142(3):591–619.
Article14. Preeprame S, Hayashi K, Lee JB, Sankawa U, Hayashi T. A novel antivirally active fucan sulfate derived from an edible brown alga, Sargassum horneri. Chem Pharm Bull (Tokyo). 2001; 49(4):484–485.
Article15. Matsumura Y. Nutrition trends in Japan. Asia Pac J Clin Nutr. 2001; 10:Suppl. S40–S47.
Article16. McHugh DJ. A guide to the seaweed industry. Rome: Food and Agriculture Organization of the United Nations;2003.17. Cardoso SM, Pereira OR, Seca AM, Pinto DC, Silva AM. Seaweeds as preventive agents for cardiovascular diseases: from nutrients to functional foods. Mar Drugs. 2015; 13(11):6838–6865.
Article18. Yamaguchi M. Regulatory mechanism of food factors in bone metabolism and prevention of osteoporosis. Yakugaku Zasshi. 2006; 126(11):1117–1137.
Article19. Shao P, Chen X, Sun P. Chemical characterization, antioxidant and antitumor activity of sulfated polysaccharide from Sargassum horneri. Carbohydr Polym. 2014; 105:260–269.
Article20. Shao P, Liu J, Chen X, Fang Z, Sun P. Structural features and antitumor activity of a purified polysaccharide extracted from Sargassum horneri. Int J Biol Macromol. 2015; 73:124–130.
Article21. Kim DS, Sung NY, Park SY, Kim G, Eom J, Yoo JG, et al. Immunomodulating activity of Sargassum horneri extracts in RAW264.7 macrophages. J Nutr Health. 2018; 51(6):507–514.
Article22. Kang BK, Kim KBWR, Ahn NK, Choi YU, Kim MJ, Bark SW, et al. Immuno-stimulating activities of skipjack tuna Katsuwonus pelamis cooking juice concentrates on mouse macrophages and spleen cells. Korean J Fish Aquat Sci. 2014; 47(6):776–784.
Article23. Shan BE, Yoshida Y, Kuroda E, Yamashita U. Immunomodulating activity of seaweed extract on human lymphocytes in vitro. Int J Immunopharmacol. 1999; 21(1):59–70.24. Mischell BB, Shiigi SM. Normal peritoneal cells. In : Mischell BB, Shiigi SM, editors. Selected Methods in Cellular Immunology. San Fransisco (CA): W. H. Freman and Company;1980. p. 156.25. Kim P, Ko SK, Pyo MY. Effects of hot water extract of chaga mushroom on the proliferation and cytokines production of mouse splenocytes in vitro. Yakhak Hoeji. 2010; 54(3):187–191.26. Lee JK, Lee MK, Yun YP, Kim Y, Kim JS, Kim YS, et al. Acemannan purified from Aloe vera induces phenotypic and functional maturation of immature dendritic cells. Int Immunopharmacol. 2001; 1(7):1275–1284.
Article27. Kim BH, Cho DH, Cho JY. Modulatory effect of kaempferitrin, a 3,7-diglycosylflavone, on the LPS-mediated up-regulation of surface co-stimulatory molecules and CD29-mediated cell-cell adhesion in monocytic- and macrophage-like cells. Yakhak Hoeji. 2007; 51(6):482–489.28. Virchow JC Jr, Oehling A, Boer L, Hansel TT, Werner P, Matthys H, et al. Pulmonary function, activated T cells, peripheral blood eosinophilia, and serum activity for eosinophil survival in vitro: a longitudinal study in bronchial asthma. J Allergy Clin Immunol. 1994; 94(2 Pt 1):240–249.
Article29. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995; 155(3):1151–1164.30. Byun MW, Byun EH. Immunological synergistic effects of combined treatment with herbal preparation (HemoHIM) and red ginseng extracts. J Korean Soc Food Sci Nutr. 2015; 44(2):182–190.
Article31. Chiou WF, Chou CJ, Chen CF. Camptothecin suppresses nitric oxide biosynthesis in RAW 264.7 macrophages. Life Sci. 2001; 69(6):625–635.
Article32. Hibbs JB Jr, Taintor RR, Vavrin Z, Rachlin EM. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988; 157(1):87–94.
Article33. Pyo SN, Son EH. Introduction to immunology. Seoul: Shinil Books;2008.34. Lee GN, Kwon OH. Clinical laboratory file. 3rd edition. Seoul: Medical Publisher;2003.35. Pisa P, Halapi E, Pisa EK, Gerdin E, Hising C, Bucht A, et al. Selective expression of interleukin 10, interferon gamma, and granulocyte-macrophage colony-stimulating factor in ovarian cancer biopsies. Proc Natl Acad Sci U S A. 1992; 89(16):7708–7712.
Article36. Sypek JP, Chung CL, Mayor SE, Subramanyam JM, Goldman SJ, Sieburth DS, et al. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993; 177(6):1797–1802.
Article37. Lee JW, Jang MH, Choi JS, Ahn TW. The effect of Yongyukjowitang distillate on the immune activity of spleen cells of aged rats. J Sasang Const Med. 2013; 25(3):218–232.38. Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999; 17(1):189–220.
Article39. Wang R, Jaw JJ, Stutzman NC, Zou Z, Sun PD. Natural killer cell-produced IFN-γ and TNF-α induce target cell cytolysis through up-regulation of ICAM-1. J Leukoc Biol. 2012; 91(2):299–309.
Article40. Frese-Schaper M, Keil A, Yagita H, Steiner SK, Falk W, Schmid RA, et al. Influence of natural killer cells and perforinmediated cytolysis on the development of chemically induced lung cancer in A/J mice. Cancer Immunol Immunother. 2014; 63(6):571–580.
Article41. Li Q, Morimoto K, Nakadai A, Inagaki H, Katsumata M, Shimizu T, et al. Forest bathing enhances human natural killer activity and expression of anti-cancer proteins. Int J Immunopathol Pharmacol. 2007; 20:2 suppl 2. 3–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunomodulating activity of Sargassum horneri extracts in RAW264.7 macrophages
- Inhibitory effects of Sargassum horneri extract against endoplasmic reticulum stress in HepG2 cells
- Antioxidant and immune-enhancing effects of Fuyu persimmons and Hachiya persimmons
- Enhancing Effect of Zingiber Officinale Roscoe Extracts on Mouse Spleen and Macrophage Cells Activation
- Anti-inflammatory activity of a sulfated polysaccharide isolated from an enzymatic digest of brown seaweed Sargassum horneri in RAW 264.7 cells