Immune Netw.
2019 Dec;19(6):e41. 10.4110/in.2019.19.e41.
A Critical Role of PCSK9 in Mediating IL-17-Producing T Cell Responses in Hyperlipidemia
- Affiliations
-
- 1Center for Immunology and Autoimmune Diseases, The Brown Foundation Institute of Molecular Medicine, McGovern Medical School, The University of Texas Health Science Center at Houston, Houston, TX 77030, USA.
- 2Department of Internal Medicine, McGovern Medical School, The University of Texas Health Science Center at Houston, Houston, TX 77030, USA.
- 3Center for Human Genetics, The Brown Foundation Institute of Molecular Medicine, McGovern Medical School, The University of Texas Health Science Center at Houston, Houston, TX 77030, USA. babie.teng@uth.tmc.edu
- 4Graduate School of Biomedical Sciences, The University of Texas Health Science Center at Houston, Houston, TX 77030, USA.
- KMID: 2466611
- DOI: http://doi.org/10.4110/in.2019.19.e41
Abstract
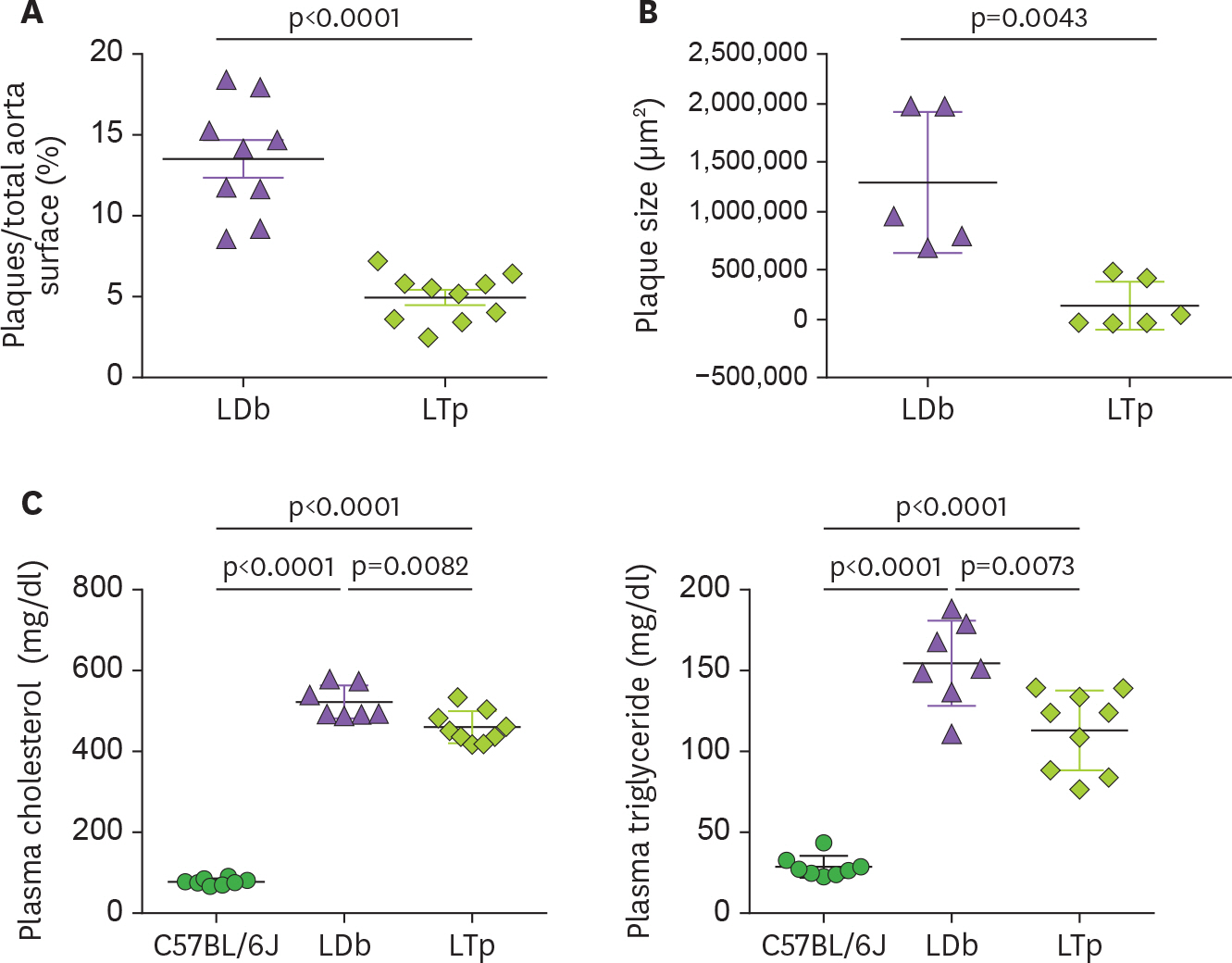
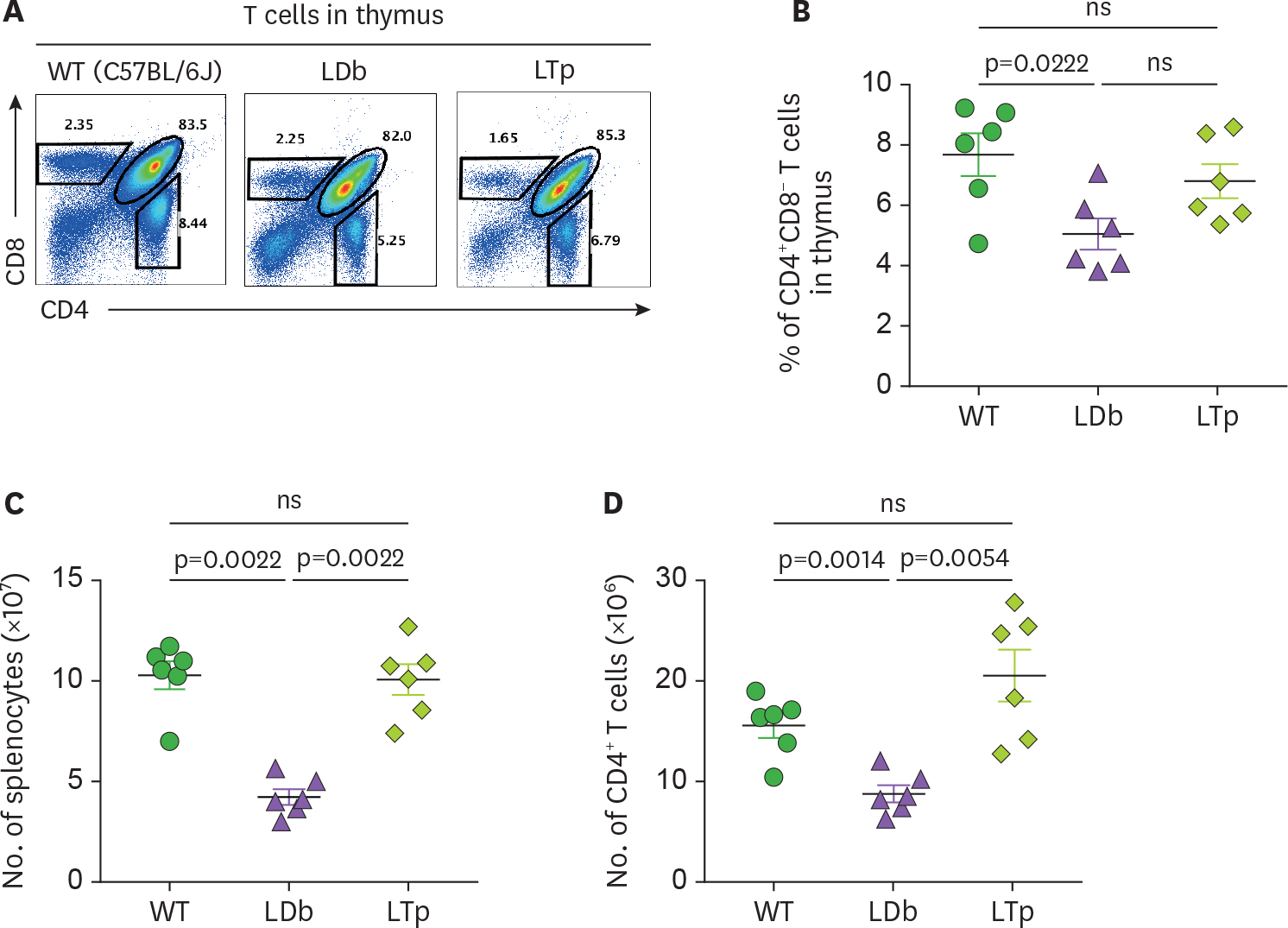
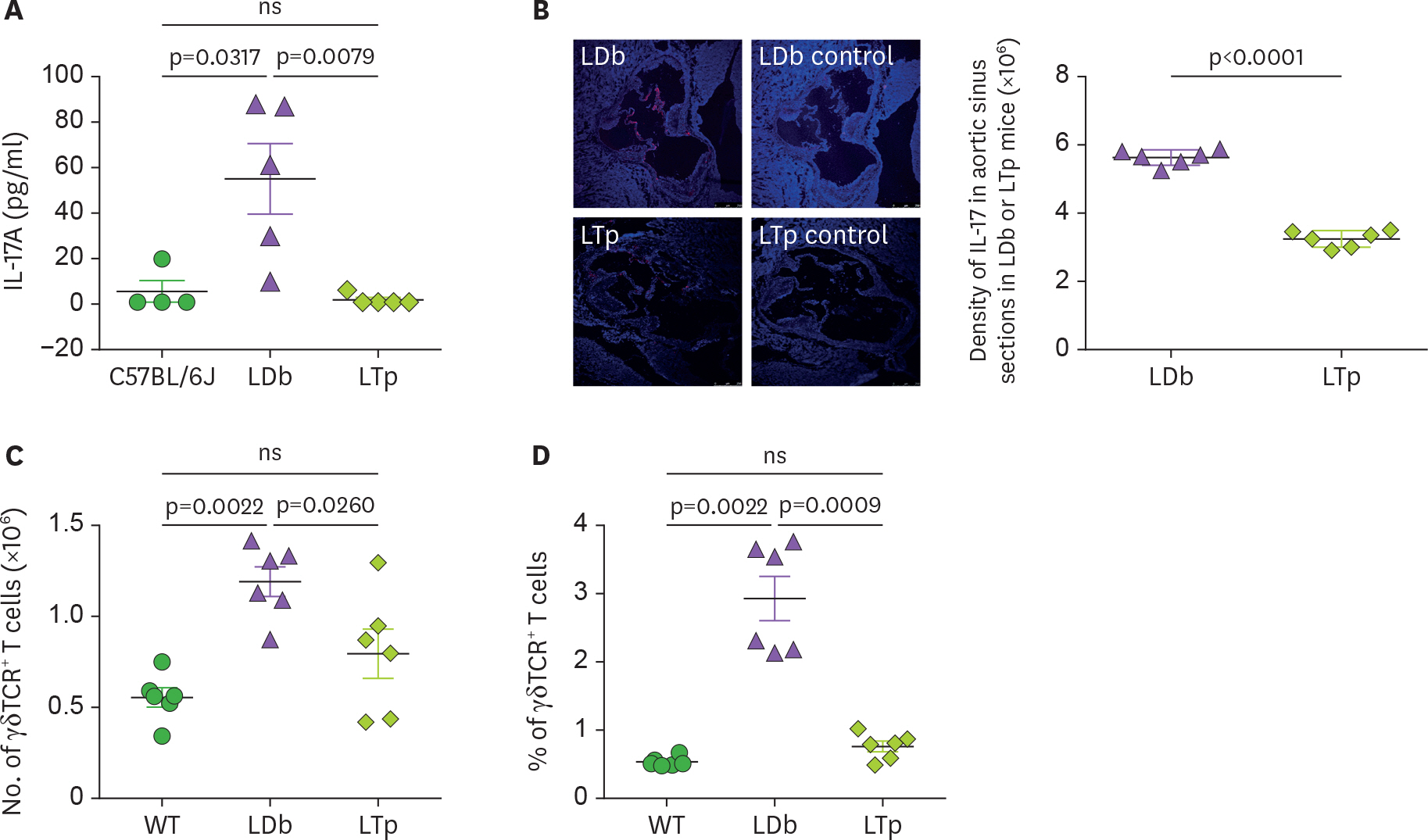
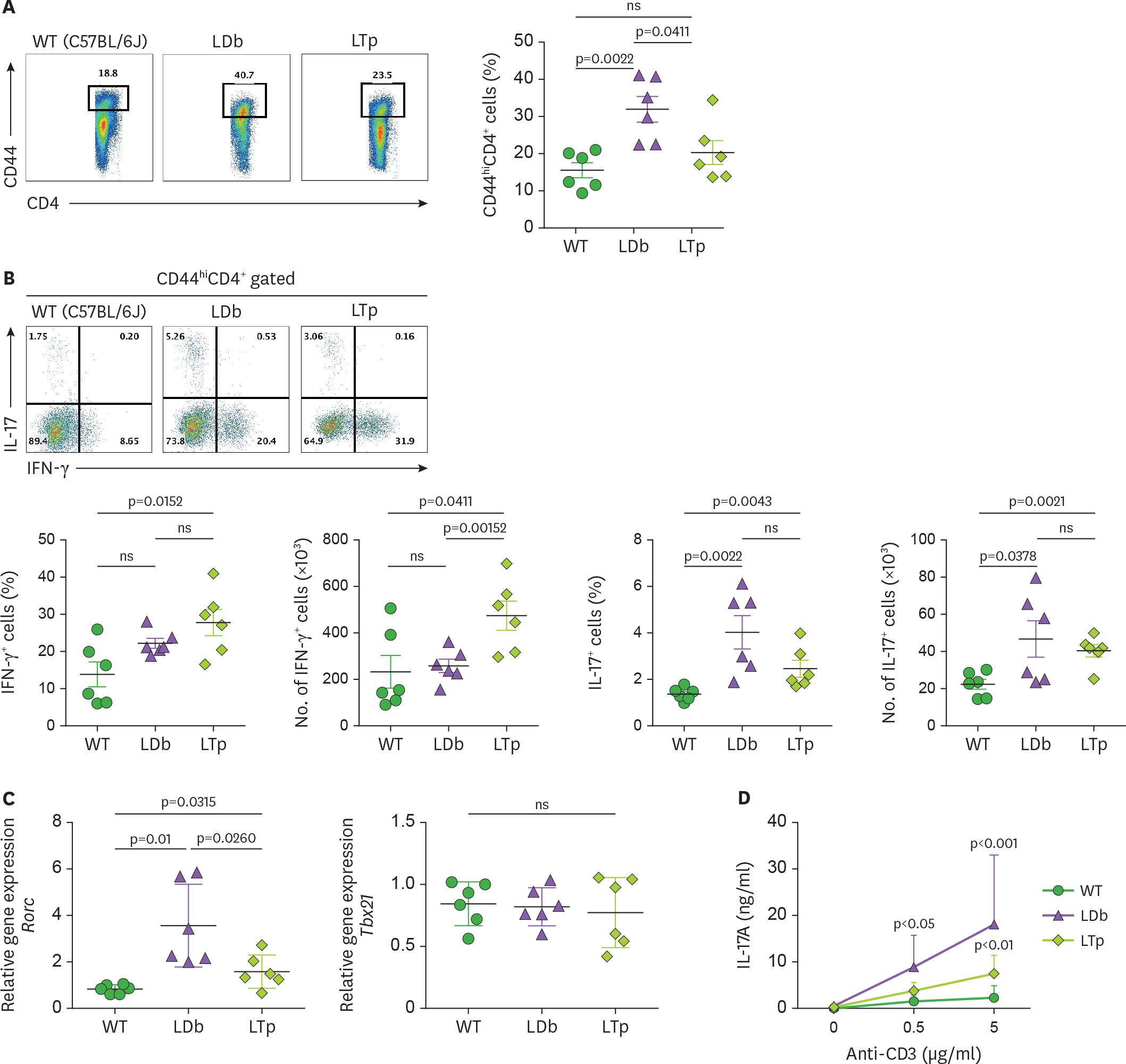
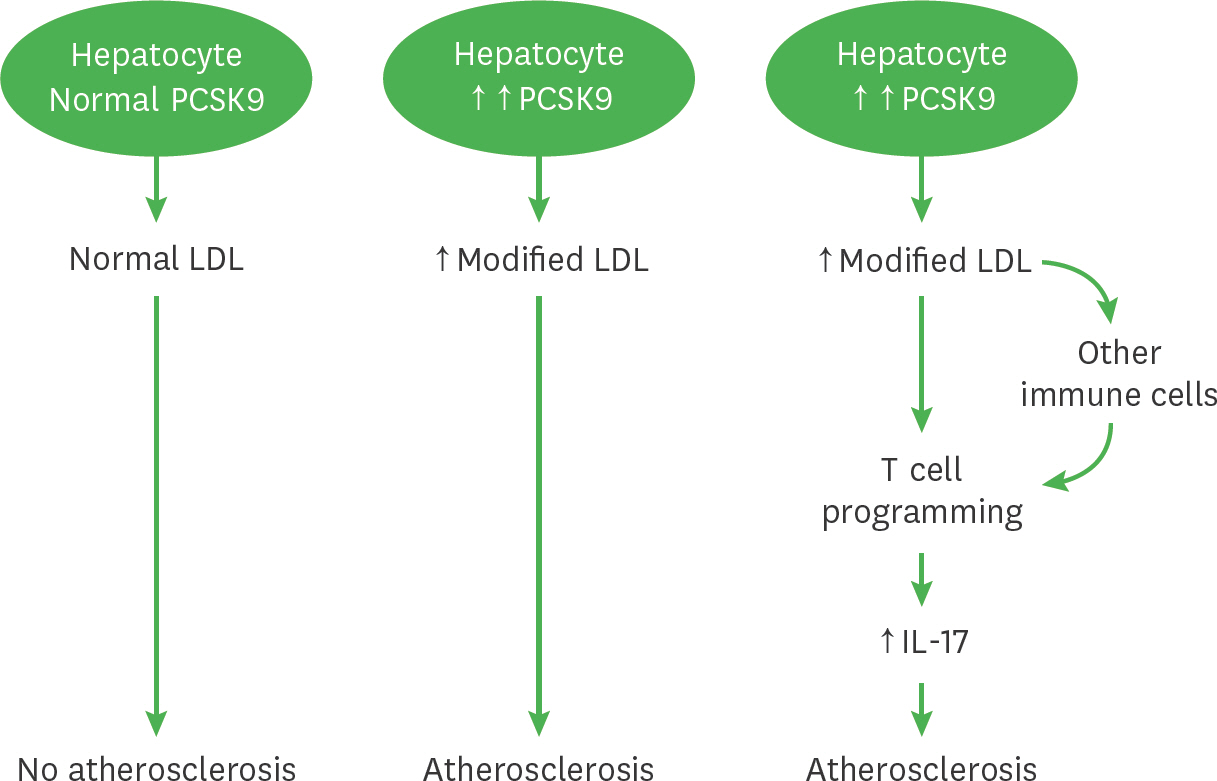
- We previously demonstrated that atherogenic Ldlr(−/−)Apobec1(−/−) (LDb) double knockout mice lacking both low-density lipoprotein receptor (LDLR) and apolipoprotein B mRNA-editing catalytic polypeptide-1 (Apobec1) had increased serum IL-17 levels, with T cell programming shifted towards Th17 cells. In this study, we assessed the role of proprotein convertase subtilisin/kexin type 9 (PCSK9) in T cell programming and atherogenesis. We deleted the Pcsk9 gene from LDb mice to generate Ldlr(−/−)Apobec1(−/−)Pcsk9(−/−) (LTp) triple knockout mice. Atherosclerosis in the aortic sinus and aorta were quantitated. Lymphoid cells were analyzed by flow cytometry, ELISA and real-time PCR. Despite of dyslipidemia, LTp mice developed barely detectable atherosclerotic lesions. The IL-17, was very low in plasma and barely detectable in the aortic sinus in the LTp mice. In the spleen, the number of CD4âºCD8â» cells and splenocytes were much lower in the LDb mice than LTp mice, whereas, the IL-17-producing cells of γδTCR⺠T cells and effector memory CD4⺠T cells (CD44(hi)CD4âº) in the spleen were significantly higher in the LDb mice than in the LTp mice. The Rorc mRNA expression levels were elevated in LDb mice compared to LTp mice. When re-stimulated with an anti-CD3 Ab, CD44(hi)CD4⺠T cells from LDb mice secreted more IL-17 than those from LTp mice. T cells from LDb mice (with PCSK9) produce more IL-17 at basal and stimulated conditions when compared with LTp mice (without PCSK9). Despite the dyslipidemic profile and the lack of LDLR, atherogenesis is markedly reduced in LTp mice. These results suggest that PCSK9 is associated with changes in T cell programming that contributes to the development of atherosclerosis.
Keyword
MeSH Terms
-
Animals
Aorta
Apolipoproteins
Atherosclerosis
Dyslipidemias
Enzyme-Linked Immunosorbent Assay
Flow Cytometry
Hyperlipidemias*
Interleukin-17
Lymphocytes
Memory
Mice
Mice, Knockout
Negotiating*
Plasma
Proprotein Convertases
Real-Time Polymerase Chain Reaction
Receptors, Lipoprotein
RNA, Messenger
Sinus of Valsalva
Spleen
T-Lymphocytes
Th17 Cells
Apolipoproteins
Interleukin-17
Proprotein Convertases
RNA, Messenger
Receptors, Lipoprotein
Figure
Reference
-
References
1. Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011; 473:317–325.
Article2. Manduteanu I, Simionescu M. Inflammation in atherosclerosis: a cause or a result of vascular disorders? J Cell Mol Med. 2012; 16:1978–1990.
Article3. Hobbs HH, Brown MS, Goldstein JL. Molecular genetics of the LDL receptor gene in familial hypercholesterolemia. Hum Mutat. 1992; 1:445–466.
Article4. Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013; 38:1092–1104.
Article5. Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012; 32:2045–2051.
Article6. Wong BW, Meredith A, Lin D, McManus BM. The biological role of inflammation in atherosclerosis. Can J Cardiol. 2012; 28:631–641.
Article7. Lim H, Kim YU, Sun H, Lee JH, Reynolds JM, Hanabuchi S, Wu H, Teng BB, Chung Y. Proatherogenic conditions promote autoimmune T helper 17 cell responses in vivo. Immunity. 2014; 40:153–165.8. Danzaki K, Matsui Y, Ikesue M, Ohta D, Ito K, Kanayama M, Kurotaki D, Morimoto J, Iwakura Y, Yagita H, et al. Interleukin-17A deficiency accelerates unstable atherosclerotic plaque formation in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2012; 32:273–280.
Article9. Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, Sokol SI, Pfau S, Pober JS, Tellides G. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009; 119:1424–1432.10. Erbel C, Chen L, Bea F, Wangler S, Celik S, Lasitschka F, Wang Y, Böckler D, Katus HA, Dengler TJ. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J Immunol. 2009; 183:8167–8175.
Article11. Gisterå A, Robertson AK, Andersson J, Ketelhuth DF, Ovchinnikova O, Nilsson SK, Lundberg AM, Li MO, Flavell RA, Hansson GK. Transforming growth factor-β signaling in T cells promotes stabilization of atherosclerotic plaques through an interleukin-17-dependent pathway. Sci Transl Med. 2013; 5:196ra100.
Article12. Taleb S, Romain M, Ramkhelawon B, Uyttenhove C, Pasterkamp G, Herbin O, Esposito B, Perez N, Yasukawa H, Van Snick J, et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med. 2009; 206:2067–2077.
Article13. Maxwell KN, Fisher EA, Breslow JL. Overexpression of PCSK9 accelerates the degradation of the LDLR in a post-endoplasmic reticulum compartment. Proc Natl Acad Sci U S A. 2005; 102:2069–2074.
Article14. Park SW, Moon YA, Horton JD. Post-transcriptional regulation of low density lipoprotein receptor protein by proprotein convertase subtilisin/kexin type 9a in mouse liver. J Biol Chem. 2004; 279:50630–50638.
Article15. Sun H, Krauss RM, Chang JT, Teng BB. PCSK9 deficiency reduces atherosclerosis, apolipoprotein B secretion, and endothelial dysfunction. J Lipid Res. 2018; 59:207–223.
Article16. Sun H, Samarghandi A, Zhang N, Yao Z, Xiong M, Teng BB. Proprotein convertase subtilisin/kexin type 9 interacts with apolipoprotein B and prevents its intracellular degradation, irrespective of the low-density lipoprotein receptor. Arterioscler Thromb Vasc Biol. 2012; 32:1585–1595.
Article17. Daugherty A, Lu H, Howatt DA, Rateri DL. Modes of defining atherosclerosis in mouse models: relative merits and evolving standards. Methods Mol Biol. 2009; 573:1–15.
Article18. Daugherty A, Rateri DL. Development of experimental designs for atherosclerosis studies in mice. Methods. 2005; 36:129–138.
Article19. Paigen B, Morrow A, Holmes PA, Mitchell D, Williams RA. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis. 1987; 68:231–240.
Article20. Tangirala RK, Rubin EM, Palinski W. Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J Lipid Res. 1995; 36:2320–2328.
Article21. Dutta R, Singh U, Li TB, Fornage M, Teng BB. Hepatic gene expression profiling reveals perturbed calcium signaling in a mouse model lacking both LDL receptor and Apobec1 genes. Atherosclerosis. 2003; 169:51–62.
Article22. Mak S, Sun H, Acevedo F, Shimmin LC, Zhao L, Teng BB, Hixson JE. Differential expression of genes in the calcium-signaling pathway underlies lesion development in the LDb mouse model of atherosclerosis. Atherosclerosis. 2010; 213:40–51.
Article23. Singh U, Zhong S, Xiong M, Li TB, Sniderman A, Teng BB. Increased plasma non-esterified fatty acids and platelet-activating factor acetylhydrolase are associated with susceptibility to atherosclerosis in mice. Clin Sci (Lond). 2004; 106:421–432.
Article24. Nischal H, Sun H, Wang Y, Ford DA, Cao Y, Wei P, Teng BB. Long-term expression of apolipoprotein B mRNA-specific hammerhead ribozyme via scAAV8.2 vector inhibits atherosclerosis in mice. Mol Ther Nucleic Acids. 2013; 2:e125.
Article25. Liu A, Frostegård J. PCSK9 plays a novel immunological role in oxidized LDL-induced dendritic cell maturation and activation of T cells from human blood and atherosclerotic plaque. J Intern Med. 2018; 284:193–210.
Article26. Jin W, Dong C. IL-17 cytokines in immunity and inflammation. Emerg Microbes Infect. 2013; 2:e60.
Article27. Stepanova H, Mensikova M, Chlebova K, Faldyna M. CD4+ and γδ TCR+ T lymphocytes are sources of interleukin-17 in swine. Cytokine. 2012; 58:152–157.28. Kysenius K, Muggalla P, Mätlik K, Arumäe U, Huttunen HJ. PCSK9 regulates neuronal apoptosis by adjusting ApoER2 levels and signaling. Cell Mol Life Sci. 2012; 69:1903–1916.
Article29. Canuel M, Sun X, Asselin MC, Paramithiotis E, Prat A, Seidah NG. Proprotein convertase subtilisin/kexin type 9 (PCSK9) can mediate degradation of the low density lipoprotein receptor-related protein 1 (LRP-1). PLoS One. 2013; 8:e64145.
Article30. Shan L, Pang L, Zhang R, Murgolo NJ, Lan H, Hedrick JA. PCSK9 binds to multiple receptors and can be functionally inhibited by an EGF-A peptide. Biochem Biophys Res Commun. 2008; 375:69–73.
Article31. Demers A, Samami S, Lauzier B, Des Rosiers C, Ngo Sock ET, Ong H, Mayer G. PCSK9 induces CD36 degradation and affects long-chain fatty acid uptake and triglyceride metabolism in adipocytes and in mouse liver. Arterioscler Thromb Vasc Biol. 2015; 35:2517–2525.32. Labonté P, Begley S, Guévin C, Asselin MC, Nassoury N, Mayer G, Prat A, Seidah NG. PCSK9 impedes hepatitis C virus infection in vitro and modulates liver CD81 expression. Hepatology. 2009; 50:17–24.33. Sharotri V, Collier DM, Olson DR, Zhou R, Snyder PM. Regulation of epithelial sodium channel trafficking by proprotein convertase subtilisin/kexin type 9 (PCSK9). J Biol Chem. 2012; 287:19266–19274.
Article34. Proto JD, Doran AC, Subramanian M, Wang H, Zhang M, Sozen E, Rymond CC, Kuriakose G, D'Agati V, Winchester R, et al. Hypercholesterolemia induces T cell expansion in humanized immune mice. J Clin Invest. 2018; 128:2370–2375.
Article35. Tabas I, Lichtman AH. Monocyte-macrophages and T cells in atherosclerosis. Immunity. 2017; 47:621–634.
Article36. Momtazi-Borojeni AA, Jaafari MR, Badiee A, Sahebkar A. Long-term generation of antiPCSK9 antibody using a nanoliposome-based vaccine delivery system. Atherosclerosis. 2019; 283:69–78.
Article37. Damsker JM, Hansen AM, Caspi RR. Th1 and Th17 cells: adversaries and collaborators. Ann N Y Acad Sci. 2010; 1183:211–221.38. Hilvo M, Simolin H, Metso J, Ruuth M, Öörni K, Jauhiainen M, Laaksonen R, Baruch A. PCSK9 inhibition alters the lipidome of plasma and lipoprotein fractions. Atherosclerosis. 2018; 269:159–165.
Article39. Adam D, Heinrich M, Kabelitz D, Schütze S. Ceramide: does it matter for T cells? Trends Immunol. 2002; 23:1–4.
Article40. Surls J, Nazarov-Stoica C, Kehl M, Olsen C, Casares S, Brumeanu TD. Increased membrane cholesterol in lymphocytes diverts T-cells toward an inflammatory response. PLoS One. 2012; 7:e38733.
Article41. Yang W, Bai Y, Xiong Y, Zhang J, Chen S, Zheng X, Meng X, Li L, Wang J, Xu C, et al. Potentiating the antitumour response of CD8+ T cells by modulating cholesterol metabolism. Nature. 2016; 531:651–655.42. Gutcher I, Becher B. APC-derived cytokines and T cell polarization in autoimmune inflammation. J Clin Invest. 2007; 117:1119–1127.
Article43. Krauzová E, Krač merová J, Rossmeislová L, Mališová L, Tencerová M, Koc M, Štich V, Šiklová M. Acute hyperlipidemia initiates proinflammatory and proatherogenic changes in circulation and adipose tissue in obese women. Atherosclerosis. 2016; 250:151–157.
Article44. Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009; 27:485–517.
Article45. Mai J, Nanayakkara G, Lopez-Pastrana J, Li X, Li YF, Wang X, Song A, Virtue A, Shao Y, Shan H, et al. Interleukin-17A promotes aortic endothelial cell activation via transcriptionally and post-translationally activating p38 mitogen-activated protein kinase (MAPK) pathway. J Biol Chem. 2016; 291:4939–4954.
Article46. Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, Galkina E. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010; 121:1746–1755.
Article47. Li G, Zhang Y, Qian Y, Zhang H, Guo S, Sunagawa M, Hisamitsu T, Liu Y. Interleukin-17A promotes rheumatoid arthritis synoviocytes migration and invasion under hypoxia by increasing MMP2 and MMP9 expression through NF-κ B/HIF-1α pathway. Mol Immunol. 2013; 53:227–236.
Article48. Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003; 101:2620–2627.
Article49. Nordlohne J, Helmke A, Ge S, Rong S, Chen R, Waisman A, Haller H, von Vietinghoff S. Aggravated atherosclerosis and vascular inflammation with reduced kidney function depend on interleukin-17 receptor a and are normalized by inhibition of interleukin-17A. JACC Basic Transl Sci. 2018; 3:54–66.
Article50. Billon C, Sitaula S, Burris TP. Inhibition of RORα/γ suppresses atherosclerosis via inhibition of both cholesterol absorption and inflammation. Mol Metab. 2016; 5:997–1005.
Article51. Cheng X, Taleb S, Wang J, Tang TT, Chen J, Gao XL, Yao R, Xie JJ, Yu X, Xia N, et al. Inhibition of IL-17A in atherosclerosis. Atherosclerosis. 2011; 215:471–474.
Article52. Ge S, Hertel B, Koltsova EK, Sörensen-Zender I, Kielstein JT, Ley K, Haller H, von Vietinghoff S. Increased atherosclerotic lesion formation and vascular leukocyte accumulation in renal impairment are mediated by interleukin-17A. Circ Res. 2013; 113:965–974.
Article53. Brauner S, Jiang X, Thorlacius GE, Lundberg AM, Östberg T, Yan ZQ, Kuchroo VK, Hansson GK, Wahren-Herlenius M. Augmented Th17 differentiation in Trim21 deficiency promotes a stable phenotype of atherosclerotic plaques with high collagen content. Cardiovasc Res. 2018; 114:158–167.
Article54. Buono C, Binder CJ, Stavrakis G, Witztum JL, Glimcher LH, Lichtman AH. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc Natl Acad Sci U S A. 2005; 102:1596–1601.
Article55. Kimura T, Kobiyama K, Winkels H, Tse K, Miller J, Vassallo M, Wolf D, Ryden C, Orecchioni M, Dileepan T, et al. Regulatory CD4+ T cells recognize major histocompatibility complex class II molecule-restricted peptide epitopes of apolipoprotein B. Circulation. 2018; 138:1130–1143.56. Meng X, Yang J, Dong M, Zhang K, Tu E, Gao Q, Chen W, Zhang C, Zhang Y. Regulatory T cells in cardiovascular diseases. Nat Rev Cardiol. 2016; 13:167–179.
Article57. Proto JD, Doran AC, Gusarova G, Yurdagul A Jr, Sozen E, Subramanian M, Islam MN, Rymond CC, Du J, Hook J, et al. Regulatory T cells promote macrophage efferocytosis during inflammation resolution. Immunity. 2018; 49:666–677. e6.
Article58. Butcher MJ, Gjurich BN, Phillips T, Galkina EV. The IL-17A/IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ Res. 2012; 110:675–687.
Article59. Gaddis DE, Padgett LE, Wu R, McSkimming C, Romines V, Taylor AM, McNamara CA, Kronenberg M, Crotty S, Thomas MJ, et al. Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat Commun. 2018; 9:1095.
Article60. Nus M, Sage AP, Lu Y, Masters L, Lam BY, Newland S, Weller S, Tsiantoulas D, Raffort J, Marcus D, et al. Marginal zone B cells control the response of follicular helper T cells to a high-cholesterol diet. Nat Med. 2017; 23:601–610.
Article61. Ryu H, Lim H, Choi G, Park YJ, Cho M, Na H, Ahn CW, Kim YC, Kim WU, Lee SH, et al. Atherogenic dyslipidemia promotes autoimmune follicular helper T cell responses via IL-27. Nat Immunol. 2018; 19:583–593.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transcriptional Regulation of T Helper 17 Cell Differentiation
- IL-17-Producing Cells in Tumor Immunity: Friends or Foes?
- IL-15 in T-Cell Responses and Immunopathogenesis
- IL-17 and IL-21: Their Immunobiology and Therapeutic Potentials
- Heterogeneity of IL-22-producing Lymphoid Tissue Inducer-like Cells in Human and Mouse