Korean J Physiol Pharmacol.
2020 Jan;24(1):101-110. 10.4196/kjpp.2020.24.1.101.
Analysis of interaction between intracellular spermine and transient receptor potential canonical 4 channel: multiple candidate sites of negatively charged amino acids for the inward rectification of transient receptor potential canonical 4
- Affiliations
-
- 1Department of Physiology, College of Medicine, Seoul National University, Seoul 03080, Korea. insuk@snu.ac.kr
- 2Office of Medical Education, College of Medicine, Seoul National University, Seoul 03080, Korea.
- 3Department of Surgery, College of Medicine, Seoul National University, Seoul 03080, Korea.
- 4Department of Cell Biology, Harvard Medical School, Boston, MA 02115, USA.
- KMID: 2466574
- DOI: http://doi.org/10.4196/kjpp.2020.24.1.101
Abstract
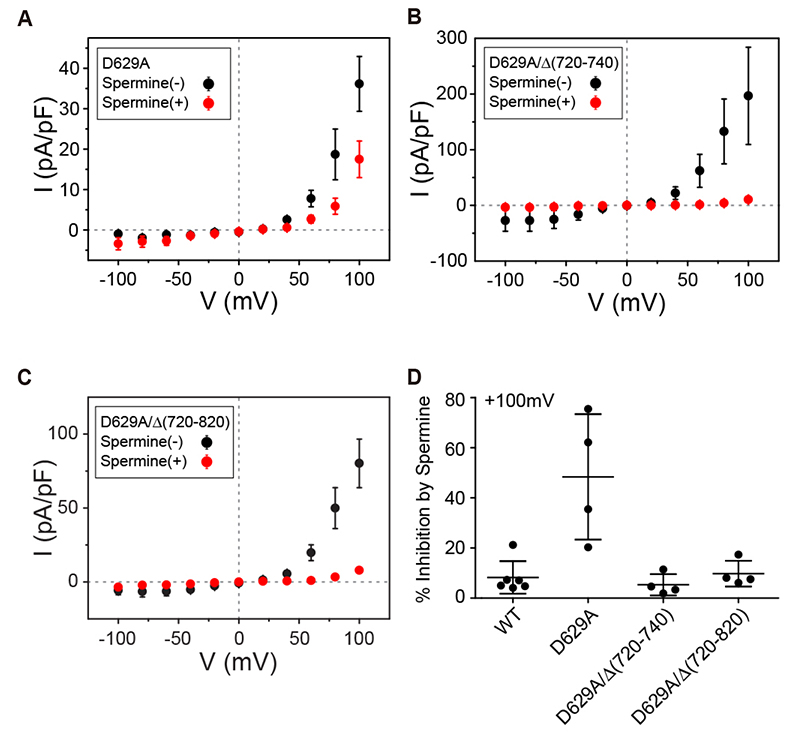
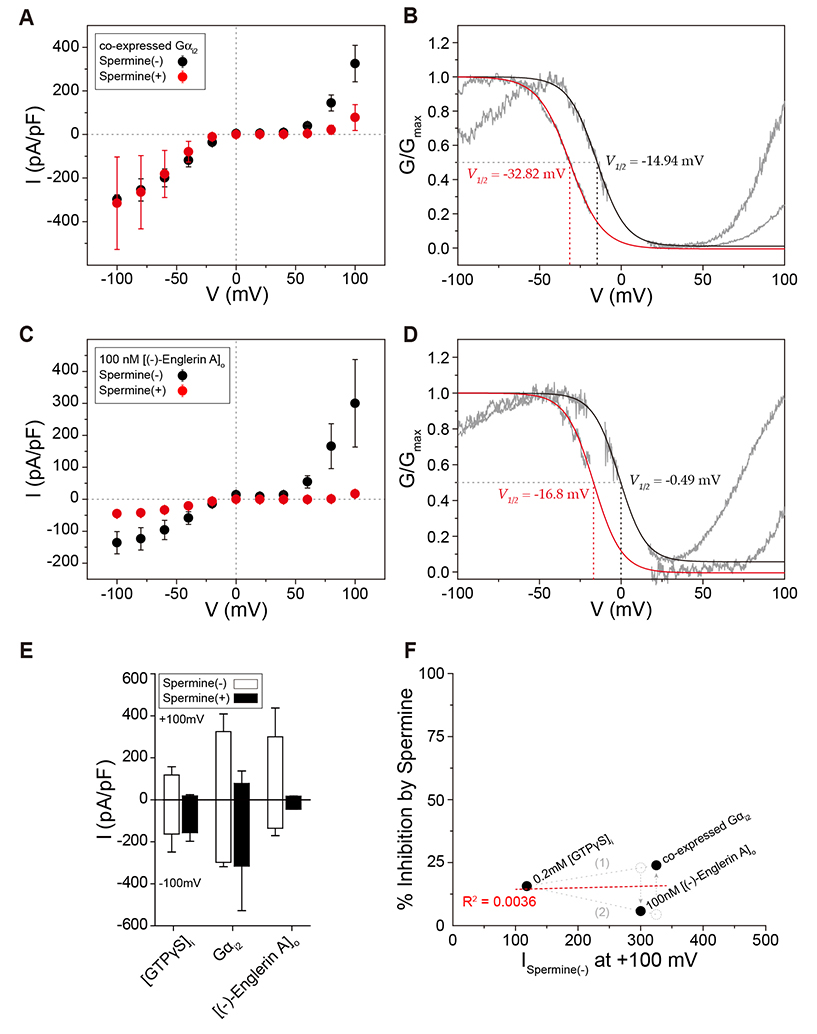
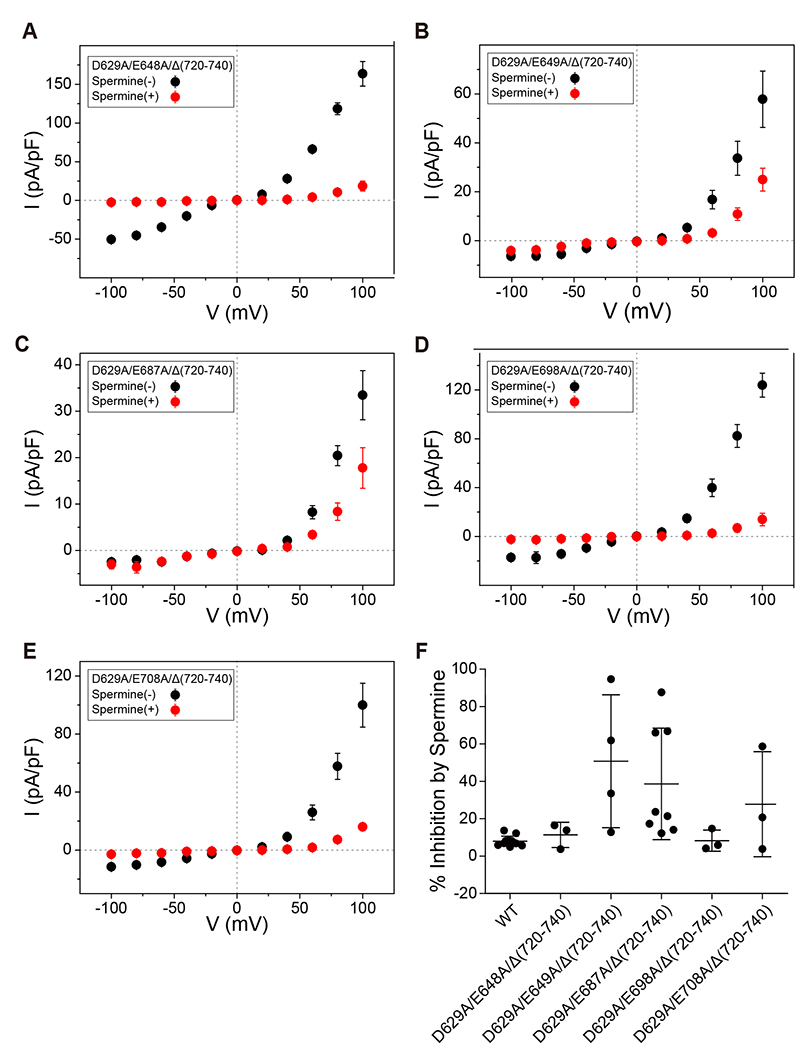
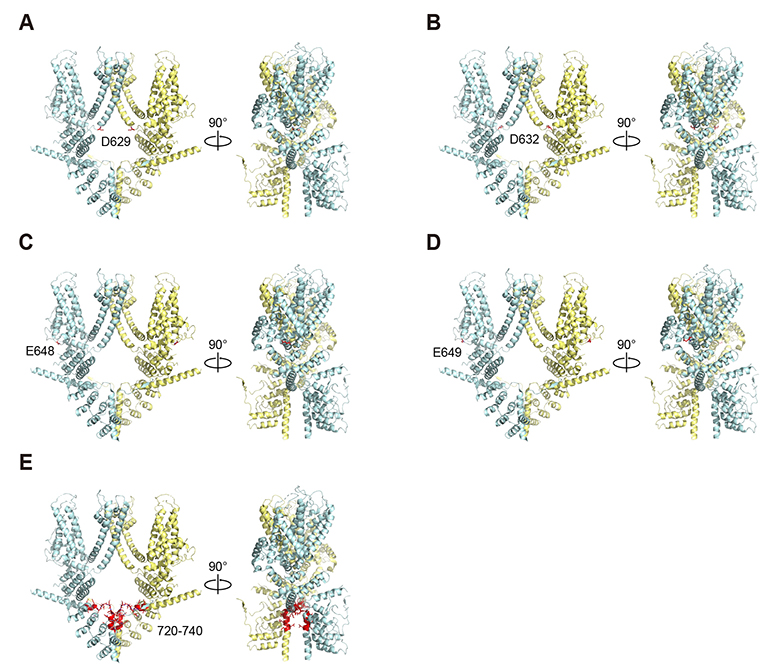
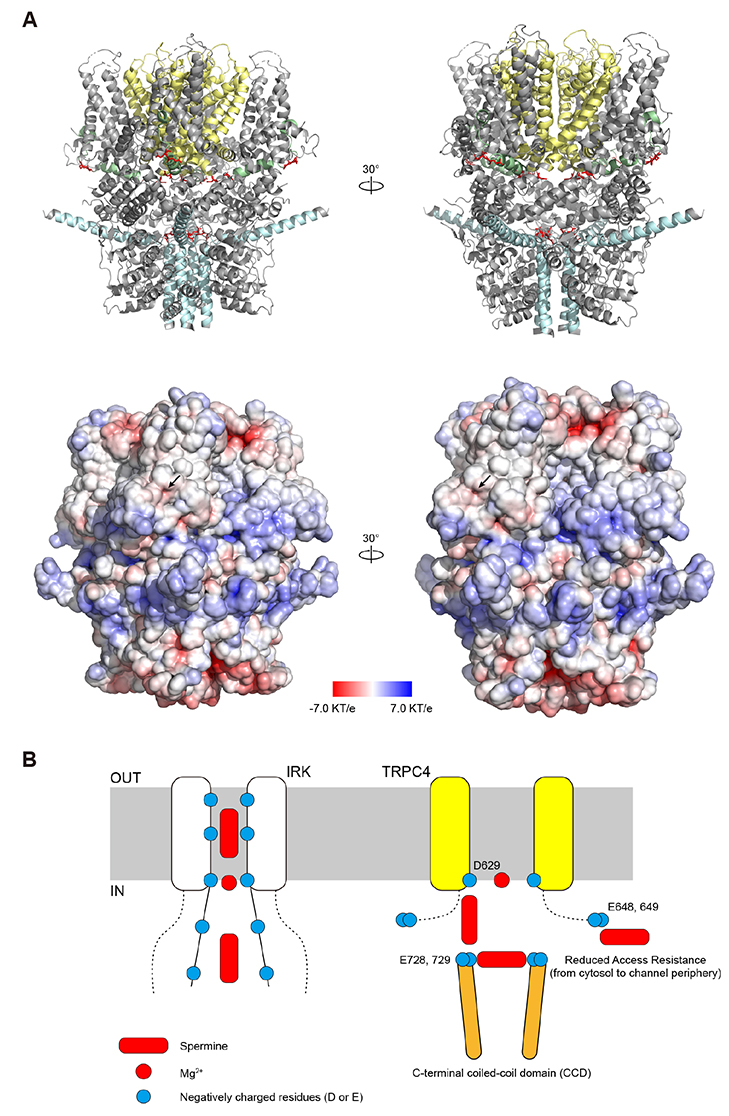
- Transient receptor potential canonical 4 (TRPC4) channel is a nonselective calcium-permeable cation channels. In intestinal smooth muscle cells, TRPC4 currents contribute more than 80% to muscarinic cationic current (mIcat). With its inward-rectifying current-voltage relationship and high calcium permeability, TRPC4 channels permit calcium influx once the channel is opened by muscarinic receptor stimulation. Polyamines are known to inhibit nonselective cation channels that mediate the generation of mIcat. Moreover, it is reported that TRPC4 channels are blocked by the intracellular spermine through electrostatic interaction with glutamate residues (E728, E729). Here, we investigated the correlation between the magnitude of channel inactivation by spermine and the magnitude of channel conductance. We also found additional spermine binding sites in TRPC4. We evaluated channel activity with electrophysiological recordings and revalidated structural significance based on Cryo-EM structure, which was resolved recently. We found that there is no correlation between magnitude of inhibitory action of spermine and magnitude of maximum current of the channel. In intracellular region, TRPC4 attracts spermine at channel periphery by reducing access resistance, and acidic residues contribute to blocking action of intracellular spermine; channel periphery, E649; cytosolic space, D629, D649, and E687.
MeSH Terms
Figure
Reference
-
1. Beech DJ. TRPC1: store-operated channel and more. Pflugers Arch. 2005; 451:53–60.
Article2. Zholos AV. TRPC5. Handb Exp Pharmacol. 2014; 222:129–156.
Article3. Kim YC, Kim SJ, Sim JH, Cho CH, Juhnn YS, Suh SH, So I, Kim KW. Suppression of the carbachol-activated nonselective cationic current by antibody against alpha subunit of Go protein in guinea-pig gastric myocytes. Pflugers Arch. 1998; 436:494–496.4. Sakamoto T, Unno T, Kitazawa T, Taneike T, Yamada M, Wess J, Nishimura M, Komori S. Three distinct muscarinic signalling pathways for cationic channel activation in mouse gut smooth muscle cells. J Physiol. 2007; 582(Pt 1):41–61.
Article5. Tsvilovskyy VV, Zholos AV, Aberle T, Philipp SE, Dietrich A, Zhu MX, Birnbaumer L, Freichel M, Flockerzi V. Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology. 2009; 137:1415–1424.6. Freichel M, Tsvilovskyy V, Camacho-Londoño JE. TRPC4- and TRPC4-containing channels. Handb Exp Pharmacol. 2014; 222:85–128.
Article7. Kim J, Ko J, Myeong J, Kwak M, Hong C, So I. TRPC1 as a negative regulator for TRPC4 and TRPC5 channels. Pflugers Arch. 2019; 471:1045–1053.
Article8. Kim J, Kwak M, Jeon JP, Myeong J, Wie J, Hong C, Kim SY, Jeon JH, Kim HJ, So I. Isoform- and receptor-specific channel property of canonical transient receptor potential (TRPC)1/4 channels. Pflugers Arch. 2014; 466:491–504.
Article9. Jeon JP, Hong C, Park EJ, Jeon JH, Cho NH, Kim IG, Choe H, Muallem S, Kim HJ, So I. Selective Gαi subunits as novel direct activators of transient receptor potential canonical (TRPC)4 and TRPC5 channels. J Biol Chem. 2012; 287:17029–17039.
Article10. Akbulut Y, Gaunt HJ, Muraki K, Ludlow MJ, Amer MS, Bruns A, Vasudev NS, Radtke L, Willot M, Hahn S, Seitz T, Ziegler S, Christmann M, Beech DJ, Waldmann H. (-)-Englerin A is a potent and selective activator of TRPC4 and TRPC5 calcium channels. Angew Chem Int Ed Engl. 2015; 54:3787–3791.11. Lopatin AN, Makhina EN, Nichols CG. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994; 372:366–369.
Article12. Lopatin AN, Makhina EN, Nichols CG. The mechanism of inward rectification of potassium channels: “long-pore plugging” by cytoplasmic polyamines. J Gen Physiol. 1995; 106:923–955.
Article13. Stanfield PR, Davies NW, Shelton PA, Sutcliffe MJ, Khan IA, Brammar WJ, Conley EC. A single aspartate residue is involved in both intrinsic gating and blockage by Mg2+ of the inward rectifier, IRK1. J Physiol. 1994; 478(Pt 1):1–6.14. Nishida M, MacKinnon R. Structural basis of inward rectification: cytoplasmic pore of the G protein-gated inward rectifier GIRK1 at 1.8 A resolution. Cell. 2002; 111:957–965.15. Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010; 90:291–366.
Article16. Pearson WL, Nichols CG. Block of the Kir2.1 channel pore by alkylamine analogues of endogenous polyamines. J Gen Physiol. 1998; 112:351–363.
Article17. Spassova M, Lu Z. Coupled ion movement underlies rectification in an inward-rectifier K+ channel. J Gen Physiol. 1998; 112:211–221.18. Obukhov AG, Nowycky MC. A cytosolic residue mediates Mg2+ block and regulates inward current amplitude of a transient receptor potential channel. J Neurosci. 2005; 25:1234–1239.19. Tsvilovskyy VV, Zholos AV, Bolton TB. Effects of polyamines on the muscarinic receptor-operated cation current in guinea-pig ileal smooth muscle myocytes. Br J Pharmacol. 2004; 143:968–975.
Article20. Kim J, Moon SH, Shin YC, Jeon JH, Park KJ, Lee KP, So I. Intracellular spermine blocks TRPC4 channel via electrostatic interaction with C-terminal negative amino acids. Pflugers Arch. 2016; 468:551–561.
Article21. Duan J, Li J, Zeng B, Chen GL, Peng X, Zhang Y, Wang J, Clapham DE, Li Z, Zhang J. Structure of the mouse TRPC4 ion channel. Nat Commun. 2018; 9:3102.
Article22. Jurrus E, Engel D, Star K, Monson K, Brandi J, Felberg LE, Brookes DH, Wilson L, Chen J, Liles K, Chun M, Li P, Gohara DW, Dolinsky T, Konecny R, Koes DR, Nielsen JE, Head-Gordon T, Geng W, Krasny R, et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci. 2018; 27:112–128.
Article23. Casero RA Jr, Marton LJ. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov. 2007; 6:373–390.
Article24. Osborne DL, Seidel ER. Gastrointestinal luminal polyamines: cellular accumulation and enterohepatic circulation. Am J Physiol. 1990; 258(4 Pt 1):G576–G584.
Article25. Swärd K, Nilsson BO, Hellstrand P. Polyamines increase Ca2+ sensitivity in permeabilized smooth muscle of guinea pig ileum. Am J Physiol. 1994; 266(6 Pt 1):C1754–C1763.26. Gomez M, Hellstrand P. Effects of polyamines on voltage-activated calcium channels in guinea-pig intestinal smooth muscle. Pflugers Arch. 1995; 430:501–507.
Article27. Nilsson BO, Hellstrand P. Effects of polyamines on intracellular calcium and mechanical activity in smooth muscle of guinea-pig taenia coli. Acta Physiol Scand. 1993; 148:37–43.
Article28. Onodera K, Unemoto T, Miyaki K, Hayashi M. Pharmacological studies on polyamines. I. Relaxing effect of spermine and spermidine on smooth muscle of guinea pig ileum contracted by 5-hydroxytryptamine and nicotine. Arch Int Pharmacodyn Ther. 1968; 174:491–494.29. Gomez M, Hellstrand P. Endogenous polyamines modulate Ca2+ channel activity in guinea-pig intestinal smooth muscle. Pflugers Arch. 1999; 438:445–451.30. Nilsson BO, Gomez MF, Swärd K, Hellstrand P. Regulation of Ca2+ channel and phosphatase activities by polyamines in intestinal and vascular smooth muscle--implications for cellular growth and contractility. Acta Physiol Scand. 2002; 176:33–41.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Negative self-regulation of transient receptor potential canonical 4 by the specific interaction with phospholipase C-δ1
- The Role of Transient Receptor Potential Channel in Pain
- Englerin A-sensing charged residues for transient receptor potential canonical 5 channel activation
- Inhibition of the Desensitization of Canonical Transient Receptor Potential Channel 5 by Dimethyl Sulfoxide
- Identification of phospholipase C β downstream effect on transient receptor potential canonical 1/4, transient receptor potential canonical 1/5 channels