Korean J Physiol Pharmacol.
2019 Sep;23(5):357-366. 10.4196/kjpp.2019.23.5.357.
Identification of phospholipase C β downstream effect on transient receptor potential canonical 1/4, transient receptor potential canonical 1/5 channels
- Affiliations
-
- 1Department of Physiology, Seoul National University College of Medicine, Seoul 03080, Korea. insuk@snu.ac.kr
- 2Department of Physiology and Biophysics, University of Washington School of Medicine, Seattle, WA 98195, USA.
- KMID: 2455812
- DOI: http://doi.org/10.4196/kjpp.2019.23.5.357
Abstract
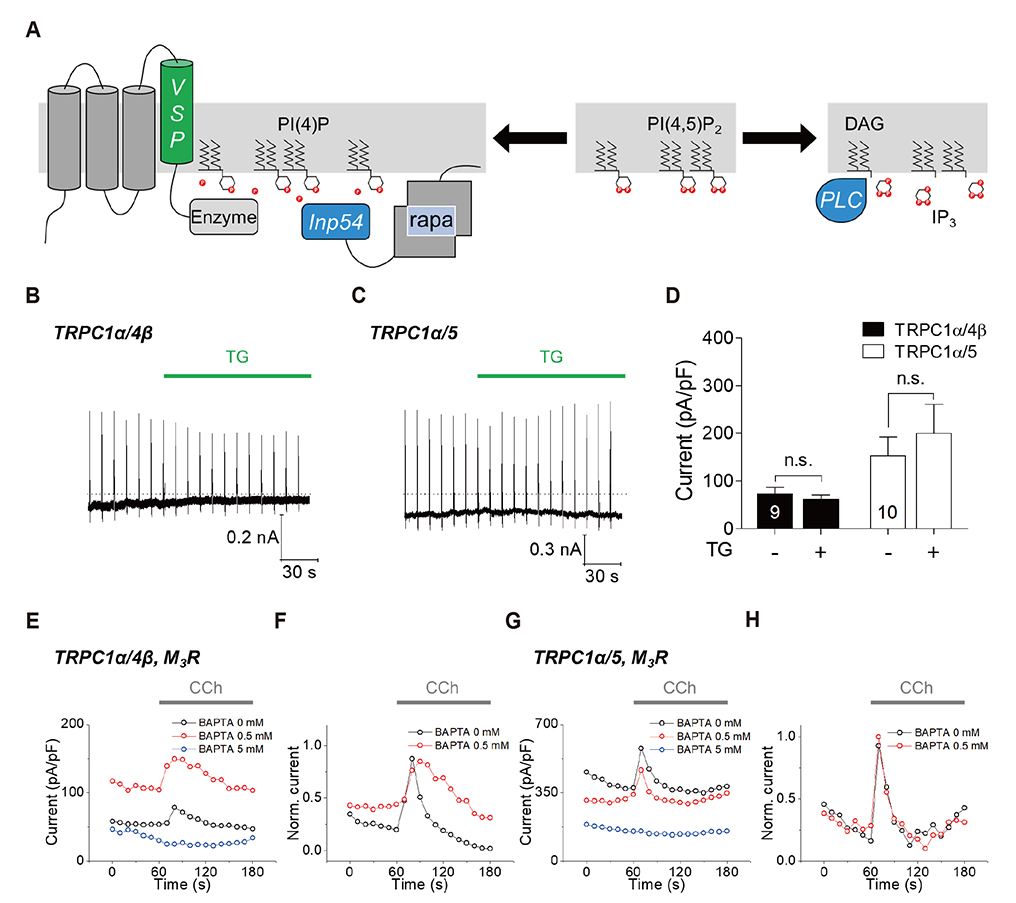
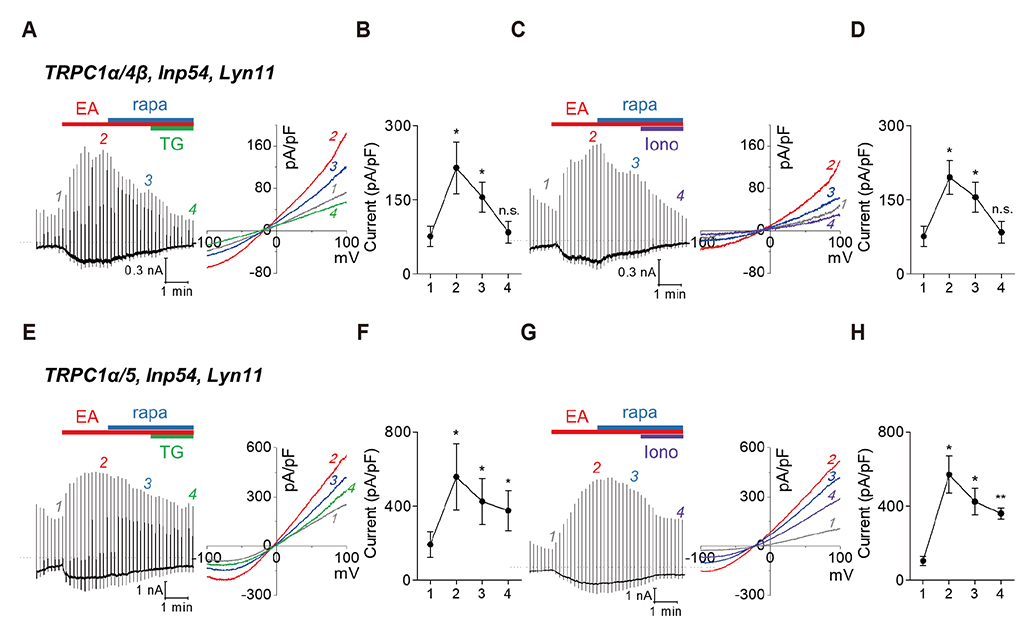
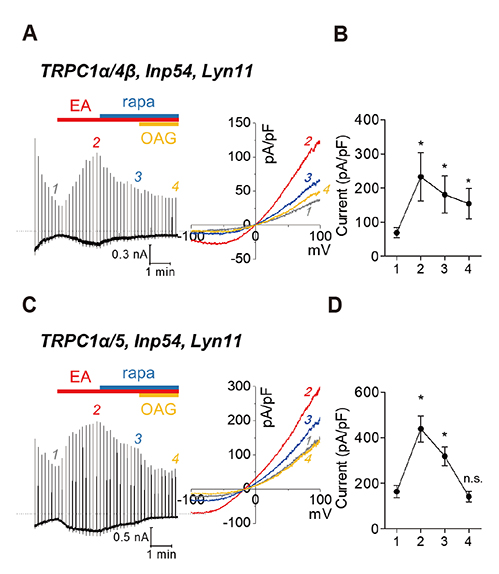
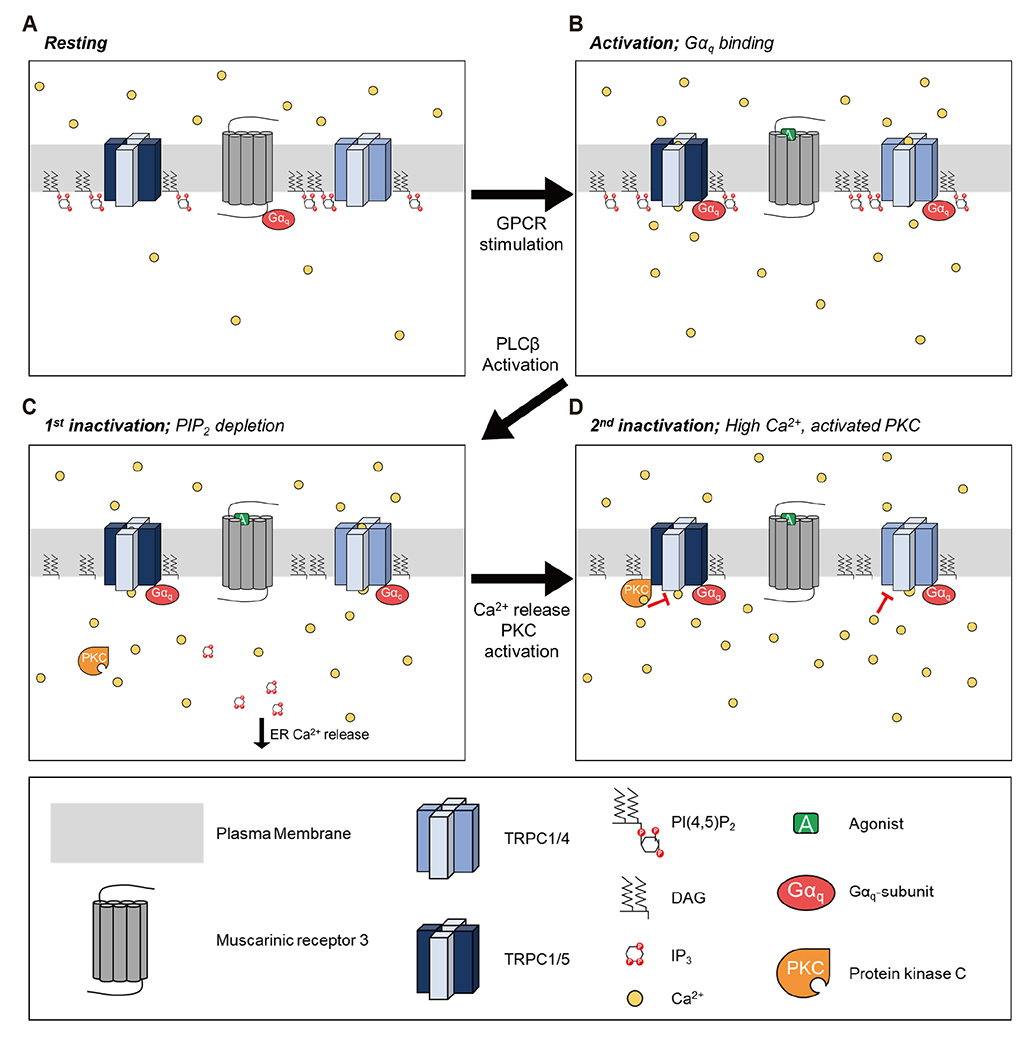
- Gα(q)-coupled receptor stimulation was implied in the activation process of transient receptor potential canonical (TRPC)1/4 and TRPC1/5 heterotetrameric channels. The inactivation occurs due to phosphatidylinositol 4,5-biphosphate (PI(4,5)Pâ‚‚) depletion. When PI(4,5)Pâ‚‚ depletion was induced by muscarinic stimulation or inositol polyphosphate 5-phosphatase (Inp54p), however, the inactivation by muscarinic stimulation was greater compared to that by Inp54p. The aim of this study was to investigate the complete inactivation mechanism of the heteromeric channels upon Gα(q)-phospholipase C β (Gα(q)-PLCβ) activation. We evaluated the activity of heteromeric channels with electrophysiological recording in HEK293 cells expressing TRPC channels. TRPC1/4 and TRPC1/5 heteromers undergo further inhibition in PLCβ activation and calcium/protein kinase C (PKC) signaling. Nevertheless, the key factors differ. For TRPC1/4, the inactivation process was facilitated by Ca²âº release from the endoplasmic reticulum, and for TRPC1/5, activation of PKC was concerned mostly. We conclude that the subsequent increase in cytoplasmic Ca²âº due to Ca²âº release from the endoplasmic reticulum and activation of PKC resulted in a second phase of channel inhibition following PI(4,5)Pâ‚‚ depletion.
Keyword
MeSH Terms
-
Calcium
Cytoplasm
Endoplasmic Reticulum
GTP-Binding Proteins
HEK293 Cells
Inositol
Phosphatidylinositol 4,5-Diphosphate
Phospholipases*
Phosphotransferases
Protein Kinase C
Transient Receptor Potential Channels
Type C Phospholipases*
Calcium
GTP-Binding Proteins
Inositol
Phosphatidylinositol 4,5-Diphosphate
Phospholipases
Phosphotransferases
Protein Kinase C
Transient Receptor Potential Channels
Type C Phospholipases
Figure
Cited by 1 articles
-
Negative self-regulation of transient receptor potential canonical 4 by the specific interaction with phospholipase C-δ1
Juyeon Ko, Jinhyeong Kim, Jongyun Myeong, Misun Kwak, Insuk So
Korean J Physiol Pharmacol. 2023;27(2):187-196. doi: 10.4196/kjpp.2023.27.2.187.
Reference
-
1. Thakur DP, Tian JB, Jeon J, Xiong J, Huang Y, Flockerzi V, Zhu MX. Critical roles of Gi/o proteins and phospholipase C-δ1 in the activation of receptor-operated TRPC4 channels. Proc Natl Acad Sci U S A. 2016; 113:1092–1097.2. Ong HL, de Souza LB, Ambudkar IS. Role of TRPC channels in store-operated calcium entry. Adv Exp Med Biol. 2016; 898:87–109.
Article3. Kim H, Jeon JP, Hong C, Kim J, Myeong J, Jeon JH, So I. An essential role of PI(4,5)P2 for maintaining the activity of the transient receptor potential canonical (TRPC)4β. Pflugers Arch. 2013; 465:1011–1021.4. Imai Y, Itsuki K, Okamura Y, Inoue R, Mori MX. A self-limiting regulation of vasoconstrictor-activated TRPC3/C6/C7 channels coupled to PI(4,5)P2-diacylglycerol signalling. J Physiol. 2012; 590:1101–1119.5. Itsuki K, Imai Y, Hase H, Okamura Y, Inoue R, Mori MX. PLC-mediated PI(4,5)P2 hydrolysis regulates activation and inactivation of TRPC6/7 channels. J Gen Physiol. 2014; 143:183–201.6. Myeong J, Kwak M, Jeon JP, Hong C, Jeon JH, So I. Close spatio-association of the transient receptor potential canonical 4 (TRPC4) channel with Gαi in TRPC4 activation process. Am J Physiol Cell Physiol. 2015; 308:C879–C889.
Article7. Zhang X, Mak S, Li L, Parra A, Denlinger B, Belmonte C, Mc-Naughton PA. Direct inhibition of the cold-activated TRPM8 ion channel by Gαq. Nat Cell Biol. 2012; 14:851–858.8. Jeon JP, Hong C, Park EJ, Jeon JH, Cho NH, Kim IG, Choe H, Muallem S, Kim HJ, So I. Selective Gαi subunits as novel direct activators of transient receptor potential canonical (TRPC)4 and TRPC5 channels. J Biol Chem. 2012; 287:17029–17039.9. Shen Y, Rampino MA, Carroll RC, Nawy S. G-protein-mediated inhibition of the Trp channel TRPM1 requires the Gβγ dimer. Proc Natl Acad Sci U S A. 2012; 109:8752–8757.
Article10. Nilius B, Flockerzi V. Mammalian transient receptor potential (TRP) cation channels. Preface. Handb Exp Pharmacol. 2014; 223:v–vi.11. Myeong J, Ko J, Kwak M, Kim J, Woo J, Ha K, Hong C, Yang D, Kim HJ, Jeon JH, So I. Dual action of the Gαq-PLCβ-PI(4,5)P2 pathway on TRPC1/4 and TRPC1/5 heterotetramers. Sci Rep. 2018; 8:12117.
Article12. Dietrich A, Fahlbusch M, Gudermann T. Classical transient receptor potential 1 (TRPC1): channel or channel regulator? Cells. 2014; 3:939–962.
Article13. Tajeddine N, Zanou N, Van Schoor M, Lebacq J, Gailly P. TRPC1: subcellular localization? J Biol Chem. 2010; 285:le1. author reply le2.
Article14. Myeong J, Ko J, Hong C, Yang D, Lee KP, Jeon JH, So I. The interaction domains of transient receptor potential canonical (TRPC)1/4 and TRPC1/5 heteromultimeric channels. Biochem Biophys Res Commun. 2016; 474:476–481.
Article15. Kinoshita-Kawada M, Tang J, Xiao R, Kaneko S, Foskett JK, Zhu MX. Inhibition of TRPC5 channels by Ca2+-binding protein 1 in Xenopus oocytes. Pflugers Arch. 2005; 450:345–354.16. Ordaz B, Tang J, Xiao R, Salgado A, Sampieri A, Zhu MX, Vaca L. Calmodulin and calcium interplay in the modulation of TRPC5 channel activity. Identification of a novel C-terminal domain for calcium/calmodulin-mediated facilitation. J Biol Chem. 2005; 280:30788–30796.17. Blair NT, Kaczmarek JS, Clapham DE. Intracellular calcium strongly potentiates agonist-activated TRPC5 channels. J Gen Physiol. 2009; 133:525–546.
Article18. Gross SA, Guzmán GA, Wissenbach U, Philipp SE, Zhu MX, Bruns D, Cavalié A. TRPC5 is a Ca2+-activated channel functionally coupled to Ca2+-selective ion channels. J Biol Chem. 2009; 284:34423–34432.19. Hong C, Seo H, Kwak M, Jeon J, Jang J, Jeong EM, Myeong J, Hwang YJ, Ha K, Kang MJ, Lee KP, Yi EC, Kim IG, Jeon JH, Ryu H, So I. Increased TRPC5 glutathionylation contributes to striatal neuron loss in Huntington's disease. Brain. 2015; 138(Pt 10):3030–3047.
Article20. Hong C, Kwak M, Myeong J, Ha K, Wie J, Jeon JH, So I. Extracellular disulfide bridges stabilize TRPC5 dimerization, trafficking, and activity. Pflugers Arch. 2015; 467:703–712.
Article21. Akbulut Y, Gaunt HJ, Muraki K, Ludlow MJ, Amer MS, Bruns A, Vasudev NS, Radtke L, Willot M, Hahn S, Seitz T, Ziegler S, Christmann M, Beech DJ, Waldmann H. (-)-Englerin A is a potent and selective activator of TRPC4 and TRPC5 calcium channels. Angew Chem Int Ed Engl. 2015; 54:3787–3791.22. Hossain MI, Iwasaki H, Okochi Y, Chahine M, Higashijima S, Nagayama K, Okamura Y. Enzyme domain affects the movement of the voltage sensor in ascidian and zebrafish voltage-sensing phosphatases. J Biol Chem. 2008; 283:18248–18259.
Article23. Mitchell CA, Brown S, Campbell JK, Munday AD, Speed CJ. Regulation of second messengers by the inositol polyphosphate 5-phosphatases. Biochem Soc Trans. 1996; 24:994–1000.
Article24. Zhu MH, Chae M, Kim HJ, Lee YM, Kim MJ, Jin NG, Yang DK, So I, Kim KW. Desensitization of canonical transient receptor potential channel 5 by protein kinase C. Am J Physiol Cell Physiol. 2005; 289:C591–C600.
Article25. Putyrski M, Schultz C. Switching heterotrimeric G protein subunits with a chemical dimerizer. Chem Biol. 2011; 18:1126–1133.
Article26. Ko J, Myeong J, Yang D, So I. Calcium permeability of transient receptor potential canonical (TRPC) 4 channels measured by TRPC4-GCaMP6s. Korean J Physiol Pharmacol. 2017; 21:133–140.
Article27. Storch U, Forst AL, Philipp M, Gudermann T, Mederos y. Transient receptor potential channel 1 (TRPC1) reduces calcium permeability in heteromeric channel complexes. J Biol Chem. 2012; 287:3530–3540.
Article28. Rohacs T. Regulation of transient receptor potential channels by the phospholipase C pathway. Adv Biol Regul. 2013; 53:341–355.
Article29. Venkatachalam K, Zheng F, Gill DL. Regulation of canonical transient receptor potential (TRPC) channel function by diacylglycerol and protein kinase C. J Biol Chem. 2003; 278:29031–29040.
Article30. Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999; 397:259–263.
Article31. Storch U, Forst AL, Pardatscher F, Erdogmus S, Philipp M, Gregoritza M, Mederos Y, Gudermann T. Dynamic NHERF interaction with TRPC4/5 proteins is required for channel gating by diacylglycerol. Proc Natl Acad Sci U S A. 2017; 114:E37–E46.
Article32. Ko J, Myeong J, Shin YC, So I. Differential PI(4,5)P2 sensitivities of TRPC4, C5 homomeric and TRPC1/4, C1/5 heteromeric channels. Sci Rep. 2019; 9:1849.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Negative self-regulation of transient receptor potential canonical 4 by the specific interaction with phospholipase C-δ1
- The Role of Transient Receptor Potential Channel in Pain
- Immunohistochemical Study on the Distribution of Canonical Transient Receptor Potential Channels in Rat Cerebellum
- Canonical Transient Receptor Potential Channels and Their Link with Cardio/Cerebro-Vascular Diseases
- Analysis of interaction between intracellular spermine and transient receptor potential canonical 4 channel: multiple candidate sites of negatively charged amino acids for the inward rectification of transient receptor potential canonical 4