Korean J Physiol Pharmacol.
2019 May;23(3):191-201. 10.4196/kjpp.2019.23.3.191.
Englerin A-sensing charged residues for transient receptor potential canonical 5 channel activation
- Affiliations
-
- 1Department of Physiology, Seoul National University College of Medicine, Seoul 03080, Korea. Insuk@snu.ac.kr
- 2College of Veterinary Medicine, Chungnam National University, Daejeon 34134, Korea.
- 3Department of Bioinformatics, Korea University, Sejong 30019, Korea.
- 4Department of Biology, University of Pennsylvania, Philadelphia, PA 19104, USA.
- KMID: 2443607
- DOI: http://doi.org/10.4196/kjpp.2019.23.3.191
Abstract
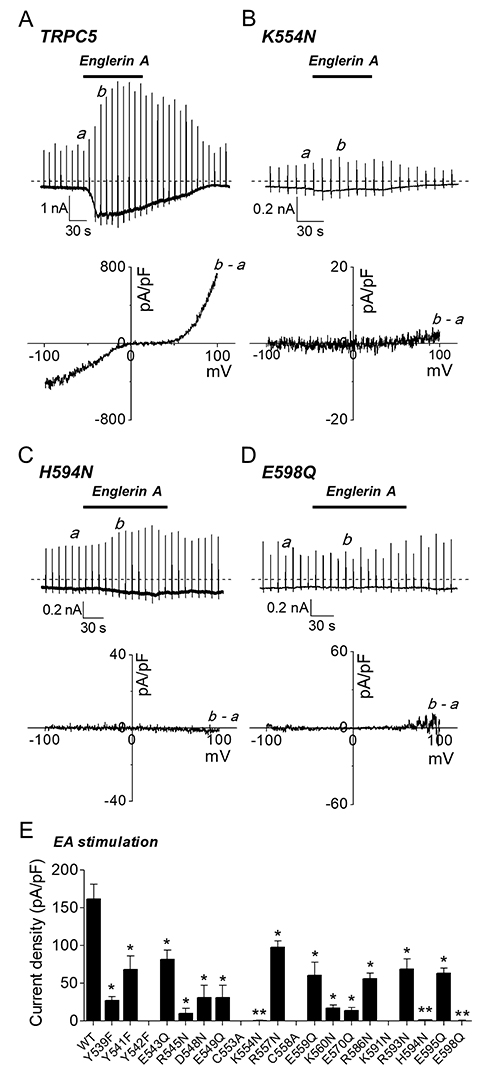
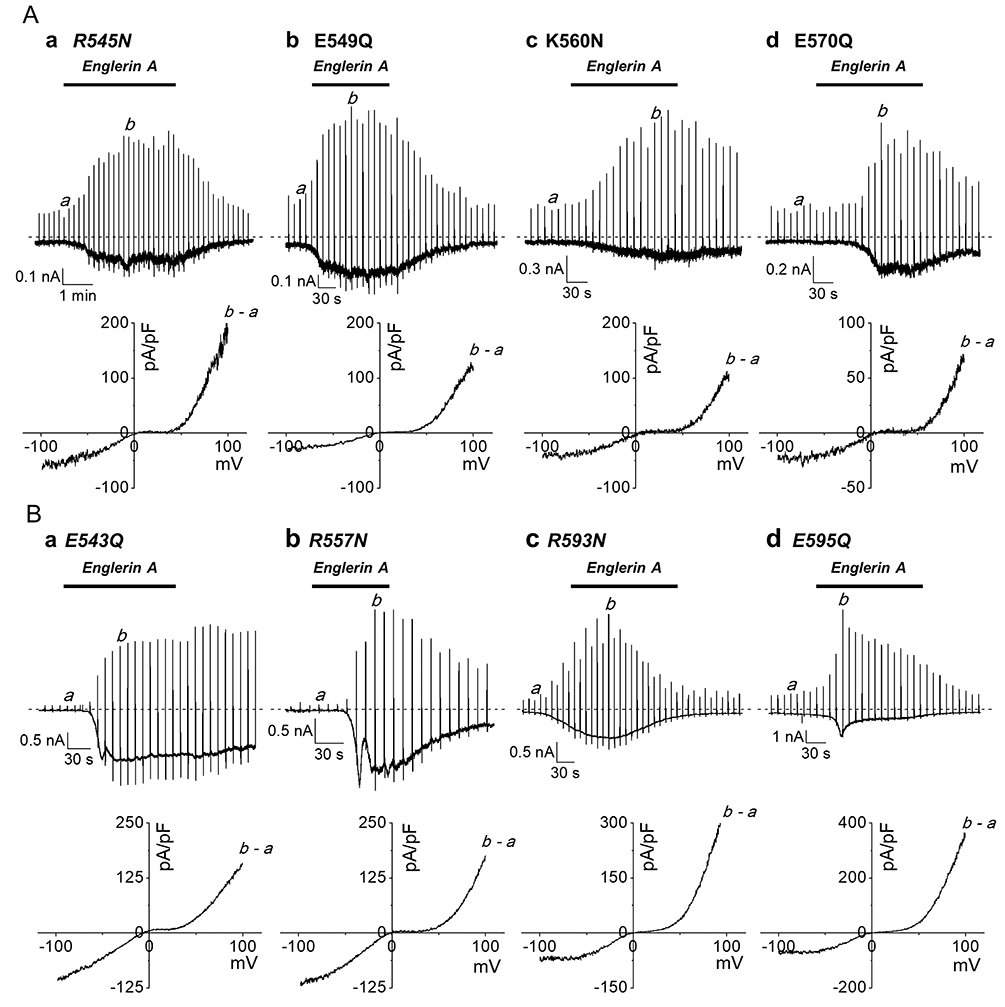
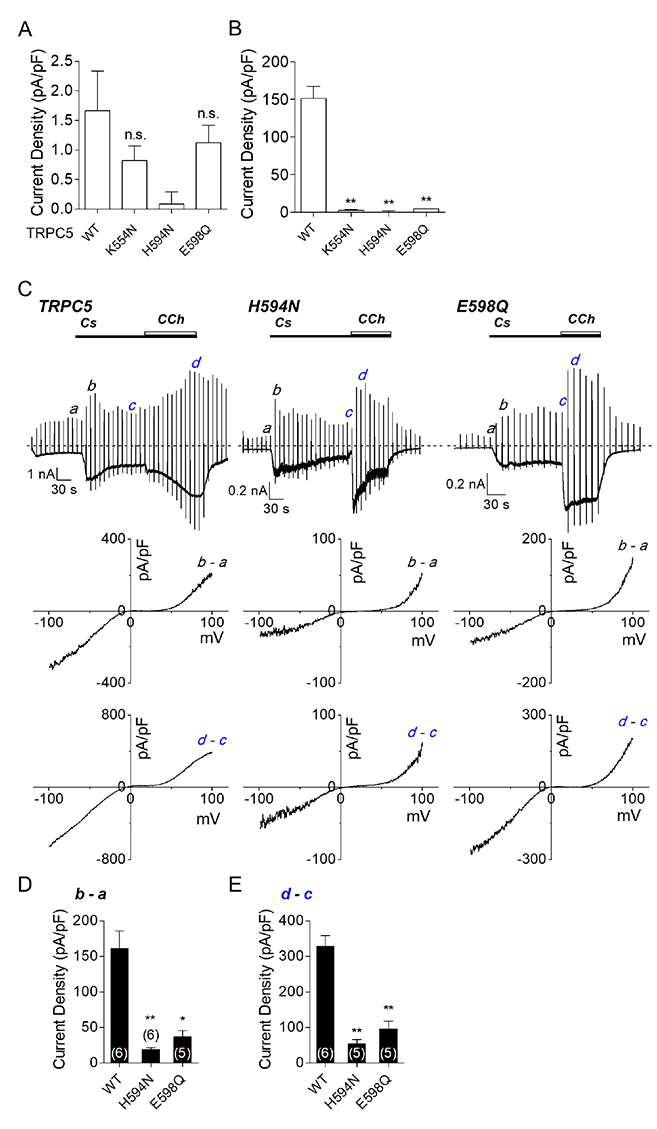
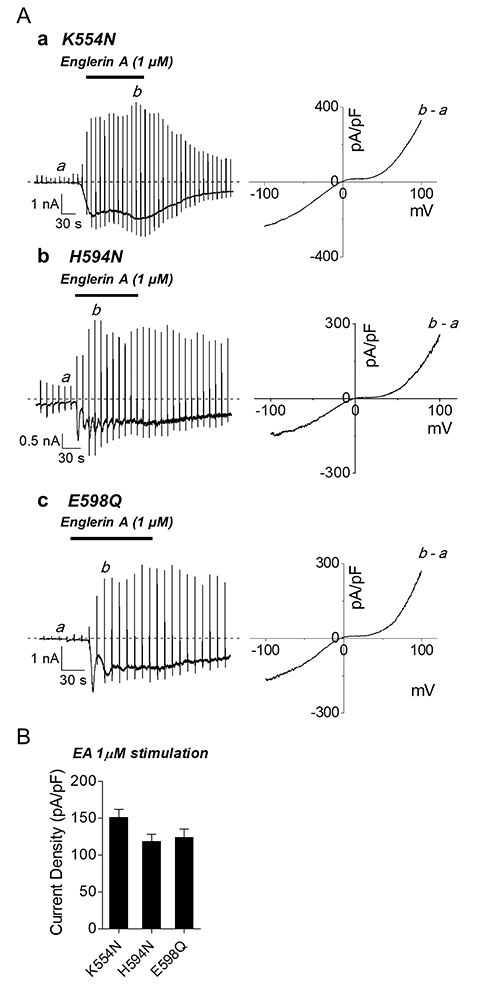
- The transient receptor potential canonical (TRPC) 5 channel, known as a nonselective cation channel, has a crucial role in calcium influx. TRPC5 has been reported to be activated by muscarinic receptor activation and extracellular pH change and inhibited by the protein kinase C pathway. Recent studies have also suggested that TRPC5 is extracellularly activated by englerin A (EA), but the mechanism remains unclear. The purpose of this study is to identify the EA-interaction sites in TRPC5 and thereby clarify the mechanism of TRPC5 activation. TRPC5 channels are over-expressed in human embryonic kidney (HEK293) cells. TRPC5 mutants were generated by site-directed mutagenesis. The whole-cell patch-clamp configuration was used to record TRPC5 currents. Western analysis was also performed to observe the expression of TRPC5 mutants. To identify the EA-interaction site in TRPC5, we first generated pore mutants. When screening the mutants with EA, we observed the EA-induced current increases of TRPC5 abolished in K554N, H594N, and E598Q mutants. The current increases of other mutants were reduced in different levels. We also examined the functional intactness of the mutants that had no effect by EA with TRPC5 agonists, such as carbachol or GTPγS. Our results suggest that the three residues, Lys-554, His-594, and Glu-598, in TRPC5 might be responsible for direct interaction with EA, inducing the channel activation. We also suggest that although other pore residues are not critical, they could partly contribute to the EA-induced channel activation.
MeSH Terms
Figure
Reference
-
1. Kim MJ, Jeon JP, Kim HJ, Kim BJ, Lee YM, Choe H, Jeon JH, Kim SJ, So I. Molecular determinant of sensing extracellular pH in classical transient receptor potential channel 5. Biochem Biophys Res Commun. 2008; 365:239–245.
Article2. Hong C, Kwak M, Myeong J, Ha K, Wie J, Jeon JH, So I. Extracellular disulfide bridges stabilize TRPC5 dimerization, trafficking, and activity. Pflugers Arch. 2015; 467:703–712.
Article3. Jeon JP, Roh SE, Wie J, Kim J, Kim H, Lee KP, Yang D, Jeon JH, Cho NH, Kim IG, Kang DE, Kim HJ, So I. Activation of TRPC4β by Gαi subunit increases Ca2+ selectivity and controls neurite morphogenesis in cultured hippocampal neuron. Cell Calcium. 2013; 54:307–319.4. Venkatachalam K, Zheng F, Gill DL. Regulation of canonical transient receptor potential (TRPC) channel function by diacylglycerol and protein kinase C. J Biol Chem. 2003; 278:29031–29040.
Article5. Sukumar P, Beech DJ. Stimulation of TRPC5 cationic channels by low micromolar concentrations of lead ions (Pb2+). Biochem Biophys Res Commun. 2010; 393:50–54.6. Hong C, Seo H, Kwak M, Jeon J, Jang J, Jeong EM, Myeong J, Hwang YJ, Ha K, Kang MJ, Lee KP, Yi EC, Kim IG, Jeon JH, Ryu H, So I. Increased TRPC5 glutathionylation contributes to striatal neuron loss in Huntington's disease. Brain. 2015; 138:3030–3047.
Article7. Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, Tominaga M, Shimizu S, Sato Y, Mori Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol. 2006; 2:596–607.
Article8. Zhu MH, Chae M, Kim HJ, Lee YM, Kim MJ, Jin NG, Yang DK, So I, Kim KW. Desensitization of canonical transient receptor potential channel 5 by protein kinase C. Am J Physiol Cell Physiol. 2005; 289:C591–C600.
Article9. Akbulut Y, Gaunt HJ, Muraki K, Ludlow MJ, Amer MS, Bruns A, Vasudev NS, Radtke L, Willot M, Hahn S, Seitz T, Ziegler S, Christmann M, Beech DJ, Waldmann H. (-)-Englerin A is a potent and selective activator of TRPC4 and TRPC5 calcium channels. Angew Chem Int Ed Engl. 2015; 54:3787–3791.10. Beck A, Speicher T, Stoerger C, Sell T, Dettmer V, Jusoh SA, Abdulmughni A, Cavalié A, Philipp SE, Zhu MX, Helms V, Wissenbach U, Flockerzi V. Conserved gating elements in TRPC4 and TRPC5 channels. J Biol Chem. 2013; 288:19471–19483.
Article11. Ko J, Myeong J, Yang D, So I. Calcium permeability of transient receptor potential canonical (TRPC) 4 channels measured by TRPC4-GCaMP6s. Korean J Physiol Pharmacol. 2017; 21:133–140.
Article12. Rodrigues T, Sieglitz F, Somovilla VJ, Cal PM, Galione A, Corzana F, Bernardes GJ. Unveiling (-)-englerin A as a modulator of L-type calcium channels. Angew Chem Int Ed Engl. 2016; 55:11077–11081.13. Okada T, Shimizu S, Wakamori M, Maeda A, Kurosaki T, Takada N, Imoto K, Mori Y. Molecular cloning and functional characterization of a novel receptor-activated TRP Ca2+ channel from mouse brain. J Biol Chem. 1998; 273:10279–10287.14. Jung S, Mühle A, Schaefer M, Strotmann R, Schultz G, Plant TD. Lanthanides potentiate TRPC5 currents by an action at extracellular sites close to the pore mouth. J Biol Chem. 2003; 278:3562–3571.
Article15. Gross SA, Guzmán GA, Wissenbach U, Philipp SE, Zhu MX, Bruns D, Cavalié A. TRPC5 is a Ca2+-activated channel functionally coupled to Ca2+-selective ion channels. J Biol Chem. 2009; 284:34423–34432.16. Philipp S, Cavalié A, Freichel M, Wissenbach U, Zimmer S, Trost C, Marquart A, Murakami M, Flockerzi V. A mammalian capacitative calcium entry channel homologous to Drosophila TRP and TRPL. EMBO J. 1996; 15:6166–6171.
Article17. Gees M, Colsoul B, Nilius B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb Perspect Biol. 2010; 2:a003962.18. Ludlow MJ, Gaunt HJ, Rubaiy HN, Musialowski KE, Blythe NM, Vasudev NS, Muraki K, Beech DJ. (-)-Englerin A-evoked cytotoxicity is mediated by Na+ influx and counteracted by Na+/K+-ATPase. J Biol Chem. 2017; 292:723–731.19. Muraki K, Ohnishi K, Takezawa A, Suzuki H, Hatano N, Muraki Y, Hamzah N, Foster R, Waldmann H, Nussbaumer P, Christmann M, Bon RS, Beech DJ. Na+ entry through heteromeric TRPC4/C1 channels mediates (-)Englerin A-induced cytotoxicity in synovial sarcoma cells. Sci Rep. 2017; 7:16988.
Article20. Duan J, Li J, Zeng B, Chen GL, Peng X, Zhang Y, Wang J, Clapham DE, Li Z, Zhang J. Structure of the mouse TRPC4 ion channel. Nat Commun. 2018; 9:3102.
Article21. Vinayagam D, Mager T, Apelbaum A, Bothe A, Merino F, Hofnagel O, Gatsogiannis C, Raunser S. Electron cryo-microscopy structure of the canonical TRPC4 ion channel. Elife. 2008; 7:e36615.
Article22. Semtner M, Schaefer M, Pinkenburg O, Plant TD. Potentiation of TRPC5 by protons. J Biol Chem. 2007; 282:33868–33878.
Article23. Carson C, Raman P, Tullai J, Xu L, Henault M, Thomas E, Yeola S, Lao J, McPate M, Verkuyl JM, Marsh G, Sarber J, Amaral A, Bailey S, Lubicka D, Pham H, Miranda N, Ding J, Tang HM, Ju H, et al. Englerin A agonizes the TRPC4/C5 cation channels to inhibit tumor cell line proliferation. PLoS One. 2015; 10:e0127498.
Article24. Rubaiy HN, Seitz T, Hahn S, Choidas A, Habenberger P, Klebl B, Dinkel K, Nussbaumer P, Waldmann H, Christmann M, Beech DJ. Identification of an (-)-englerin A analogue, which antagonizes (-)-englerin A at TRPC1/4/5 channels. Br J Pharmacol. 2018; 175:830–839.
Article25. Chen X, Li W, Riley AM, Soliman M, Chakraborty S, Stamatkin CW, Obukhov AG. Molecular determinants of the sensitivity to Gq/11-phospholipase C-dependent gating, Gd3+ potentiation, and Ca2+ permeability in the transient receptor potential canonical type 5 (TRPC5) channel. J Biol Chem. 2017; 292:898–911.26. Duan J, Li J, Chen GL, Xie K, Peng X, Zhou W, Zhong J, Zhang Y, Xu J, Xue C, Zhu L, Liu W, Tian XL, Wang J, Zeng B, Clapham DE, Li Z, Zhang J. Cryo-EM structure of the receptor-activated TRPC5 ion channel at 29 angstrom resolution. bioRxiv. 2018; 2018:467969.
Article27. Li J, Zhang X, Song X, Liu R, Zhang J, Li Z. The structure of TRPC ion channels. Cell Calcium. 2019; 80:25–28.
Article28. Kim H, Kim J, Jeon JP, Myeong J, Wie J, Hong C, Kim HJ, Jeon JH, So I. The roles of G proteins in the activation of TRPC4 and TRPC5 transient receptor potential channels. Channels (Austin). 2012; 6:333–343.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of interaction between intracellular spermine and transient receptor potential canonical 4 channel: multiple candidate sites of negatively charged amino acids for the inward rectification of transient receptor potential canonical 4
- Transient Receptor Potential Canonical 4 and 5 Channel Antagonist ML204 Depolarized Pacemaker Potentials of Interstitial Cells of Cajal
- Negative self-regulation of transient receptor potential canonical 4 by the specific interaction with phospholipase C-δ1
- Homo-dimerization of RyR1 C-terminus via charged residues in random coils or in an alpha-helix
- The Role of Transient Receptor Potential Channel in Pain