Yonsei Med J.
2016 Jan;57(1):33-40. 10.3349/ymj.2016.57.1.33.
Anti-Proliferative and Apoptotic Activities of Mullerian Inhibiting Substance Combined with Calcitriol in Ovarian Cancer Cell Lines
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 2Institute of Women's Life Medical Science, Yonsei University College of Medicine, Seoul, Korea. ytkchoi@yuhs.ac
- 3Department of Obstetrics and Gynecology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2466349
- DOI: http://doi.org/10.3349/ymj.2016.57.1.33
Abstract
- PURPOSE
This study aimed to investigate whether Mullerian inhibiting substance (MIS) in combination with calcitriol modulates proliferation and apoptosis of human ovarian cancer (OCa) cell lines (SKOV3, OVCAR3, and OVCA433) and identify the signaling pathway by which MIS mediates apoptosis.
MATERIALS AND METHODS
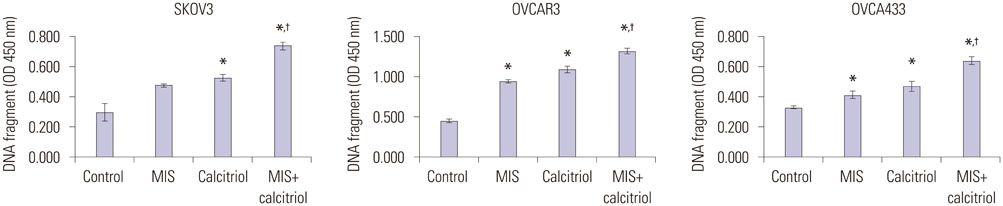
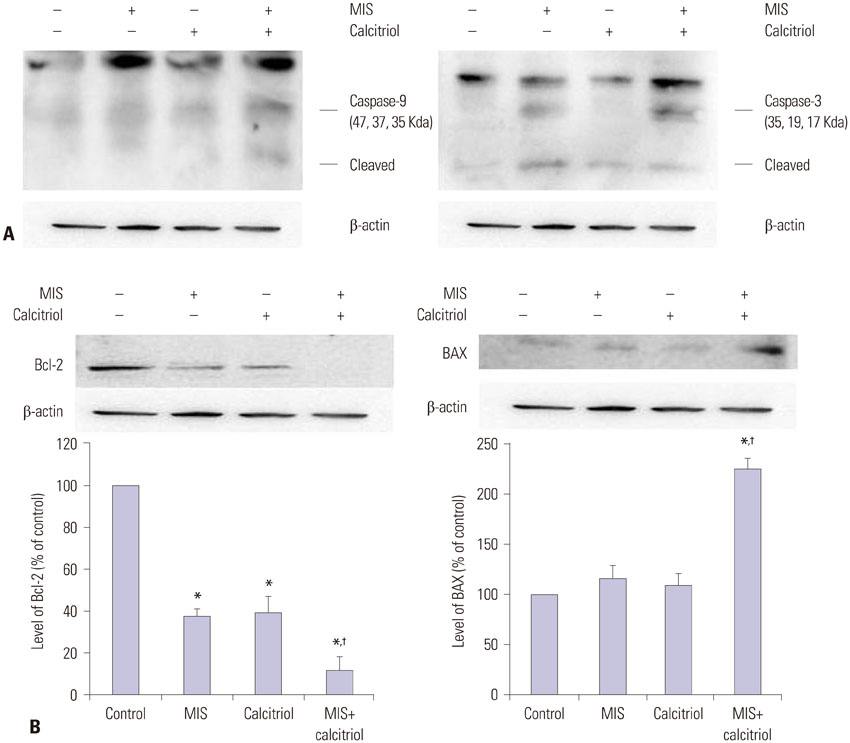
OCa cell lines were treated with MIS in the absence or presence of calcitriol. Cell viability and proliferation were evaluated using the Cell Counting Kit-8 assay and apoptosis was evaluated by DNA fragmentation assay. Western blot and enzyme-linked immunosorbent assay were used to determine the signaling pathway.
RESULTS
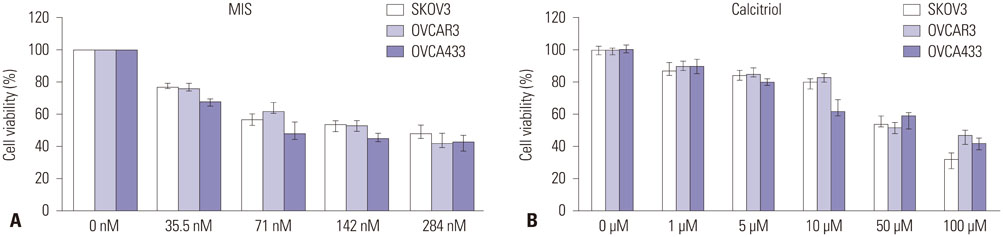
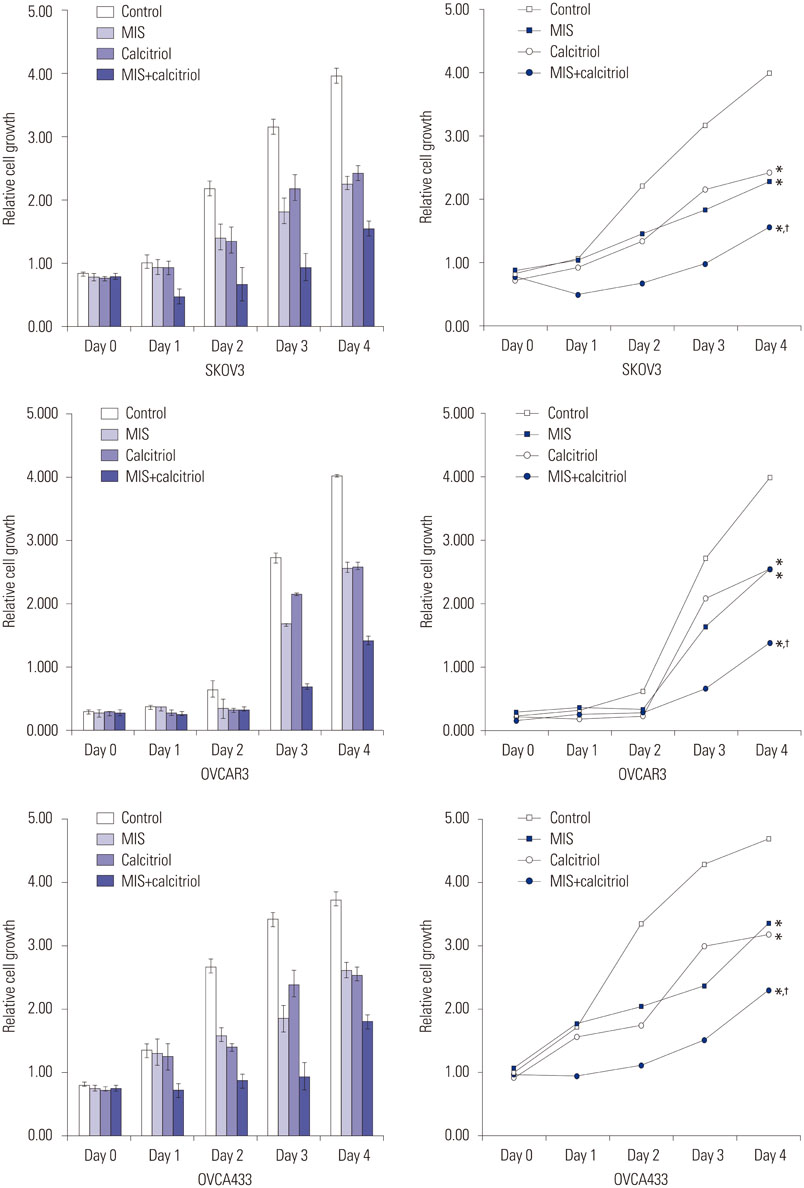
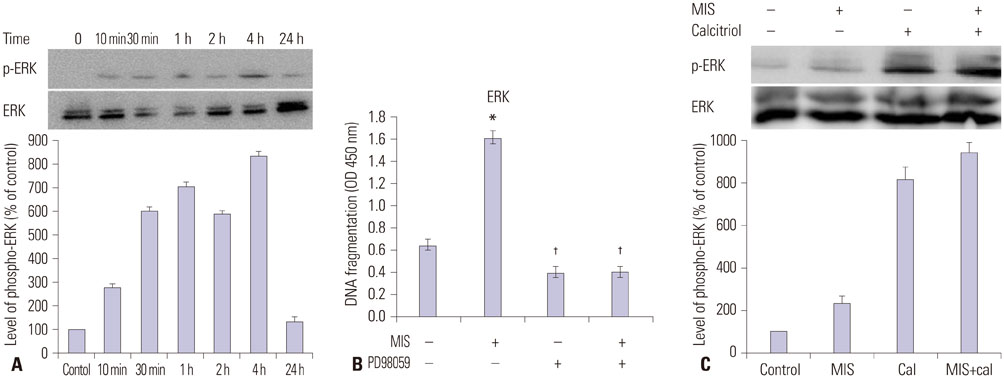
The cells showed specific staining for the MIS type II receptor. Treatment of OCa cells with MIS and calcitriol led to dose- and time-dependent inhibition of cell growth and survival. The combination treatment significantly suppressed cell growth, down-regulated the expression of B-cell lymphoma 2 (Bcl-2), and up-regulated the expressions of Bcl-2 associated X protein, caspase-3, and caspase-9 through the extracellular signal-regulated kinase signaling pathway.
CONCLUSION
These results, coupled with a much-needed decrease in the toxic side effects of currently employed therapeutic agents, provide a strong rationale for testing the therapeutic potential of MIS, alone or in combination with calcitriol, in the treatment of OCa.
MeSH Terms
-
Anti-Mullerian Hormone/*pharmacology
Apoptosis/*drug effects
Calcitriol/*pharmacology
Caspase 3/metabolism
Caspase 9/metabolism
Cell Cycle/drug effects
Cell Line, Tumor
Cell Proliferation/*drug effects
Cell Survival/drug effects
DNA Fragmentation/*drug effects
Enzyme-Linked Immunosorbent Assay
Extracellular Signal-Regulated MAP Kinases/metabolism
Female
Growth Inhibitors/metabolism/pharmacology
Humans
Ovarian Neoplasms/*drug therapy/metabolism/*pathology
Receptors, Peptide
Receptors, Transforming Growth Factor beta
Signal Transduction/*drug effects
Anti-Mullerian Hormone
Calcitriol
Caspase 3
Caspase 9
Extracellular Signal-Regulated MAP Kinases
Growth Inhibitors
Receptors, Peptide
Receptors, Transforming Growth Factor beta
Figure
Reference
-
1. Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003; 53:5–26.
Article2. Berkenblit A, Cannistra SA. Advances in the management of epithelial ovarian cancer. J Reprod Med. 2005; 50:426–438.3. Salom E, Almeida Z, Mirhashemi R. Management of recurrent ovarian cancer: evidence-based decisions. Curr Opin Oncol. 2002; 14:519–527.
Article4. MacLaughlin DT, Teixeira J, Donahoe PK. Perspective: reproductive tract development--new discoveries and future directions. Endocrinology. 2001; 142:2167–2172.
Article5. Coughlin JP, Donahoe PK, Budzik GP, MacLaughlin DT. Müllerian inhibiting substance blocks autophosphorylation of the EGF receptor by inhibiting tyrosine kinase. Mol Cell Endocrinol. 1987; 49:75–86.
Article6. Gupta V, Carey JL, Kawakubo H, Muzikansky A, Green JE, Donahoe PK, et al. Mullerian inhibiting substance suppresses tumor growth in the C3(1)T antigen transgenic mouse mammary carcinoma model. Proc Natl Acad Sci U S A. 2005; 102:3219–3224.
Article7. Segev DL, Ha TU, Tran TT, Kenneally M, Harkin P, Jung M, et al. Mullerian inhibiting substance inhibits breast cancer cell growth through an NFkappa B-mediated pathway. J Biol Chem. 2000; 275:28371–28379.
Article8. Renaud EJ, MacLaughlin DT, Oliva E, Rueda BR, Donahoe PK. Endometrial cancer is a receptor-mediated target for Mullerian Inhibiting Substance. Proc Natl Acad Sci U S A. 2005; 102:111–116.
Article9. Barbie TU, Barbie DA, MacLaughlin DT, Maheswaran S, Donahoe PK. Mullerian Inhibiting Substance inhibits cervical cancer cell growth via a pathway involving p130 and p107. Proc Natl Acad Sci U S A. 2003; 100:15601–15606.
Article10. MacLaughlin DT, Donahoe PK. Müllerian inhibiting substance/anti-Müllerian hormone: a potential therapeutic agent for human ovarian and other cancers. Future Oncol. 2010; 6:391–405.
Article11. Ponsonby AL, Lucas RM, Lewis S, Halliday J. Vitamin D status during pregnancy and aspects of offspring health. Nutrients. 2010; 2:389–407.
Article12. Giammanco M, Di Majo D, La Guardia M, Aiello S, Crescimannno M, Flandina C, et al. Vitamin D in cancer chemoprevention. Pharm Biol. 2015; 53:1399–1434.
Article13. Malloy PJ, Peng L, Wang J, Feldman D. Interaction of the vitamin D receptor with a vitamin D response element in the Mullerian-inhibiting substance (MIS) promoter: regulation of MIS expression by calcitriol in prostate cancer cells. Endocrinology. 2009; 150:1580–1587.
Article14. Krishnan AV, Moreno J, Nonn L, Malloy P, Swami S, Peng L, et al. Novel pathways that contribute to the anti-proliferative and chemopreventive activities of calcitriol in prostate cancer. J Steroid Biochem Mol Biol. 2007; 103:694–702.
Article15. Hoshiya Y, Gupta V, Segev DL, Hoshiya M, Carey JL, Sasur LM, et al. Mullerian Inhibiting Substance induces NFkB signaling in breast and prostate cancer cells. Mol Cell Endocrinol. 2003; 211:43–49.
Article16. Parry RL, Chin TW, Epstein J, Hudson PL, Powell DM, Donahoe PK. Recombinant human mullerian inhibiting substance inhibits human ocular melanoma cell lines in vitro and in vivo. Cancer Res. 1992; 52:1182–1186.17. Kim HS, Sung YJ, Paik S. Cancer Cell Line Panels Empower Genomics-Based Discovery of Precision Cancer Medicine. Yonsei Med J. 2015; 56:1186–1198.
Article18. Namkung J, Song JY, Jo HH, Kim MR, Lew YO, Donahoe PK, et al. Mullerian inhibiting substance induces apoptosis of human endometrial stromal cells in endometriosis. J Clin Endocrinol Metab. 2012; 97:3224–3230.
Article19. Leyssens C, Verlinden L, Verstuyf A. Antineoplastic effects of 1,25(OH)2D3 and its analogs in breast, prostate and colorectal cancer. Endocr Relat Cancer. 2013; 20:R31–R47.
Article20. Pereira F, Larriba MJ, Muñoz A. Vitamin D and colon cancer. Endocr Relat Cancer. 2012; 19:R51–R71.
Article21. Swami S, Krishnan AV, Feldman D. Vitamin D metabolism and action in the prostate: implications for health and disease. Mol Cell Endocrinol. 2011; 347:61–69.
Article22. Krishnan AV, Feldman D. Molecular pathways mediating the antiinflammatory effects of calcitriol: implications for prostate cancer chemoprevention and treatment. Endocr Relat Cancer. 2010; 17:R19–R38.
Article23. Gocek E, Studzinski GP. Vitamin D and differentiation in cancer. Crit Rev Clin Lab Sci. 2009; 46:190–209.
Article24. Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, Hsieh JC, et al. Molecular mechanisms of vitamin D action. Calcif Tissue Int. 2013; 92:77–98.
Article25. Kasiappan R, Shen Z, Tse AK, Jinwal U, Tang J, Lungchukiet P, et al. 1,25-Dihydroxyvitamin D3 suppresses telomerase expression and human cancer growth through microRNA-498. J Biol Chem. 2012; 287:41297–41309.
Article26. Daniel C, Schroder O, Zahn N, Gaschott T, Steinhilber D, Stein JM. The TGFbeta/Smad 3-signaling pathway is involved in butyrate-mediated vitamin D receptor (VDR)-expression. J Cell Biochem. 2007; 102:1420–1431.
Article27. Tu H, Flanders WD, Ahearn TU, Daniel CR, Gonzalez-Feliciano AG, Long Q, et al. Effects of calcium and vitamin D3 on transforming growth factors in rectal mucosa of sporadic colorectal adenoma patients: a randomized controlled trial. Mol Carcinog. 2015; 54:270–280.
Article28. Bizzarri M, Cucina A, Valente MG, Tagliaferri F, Borrelli V, Stipa F, et al. Melatonin and vitamin D3 increase TGF-beta1 release and induce growth inhibition in breast cancer cell cultures. J Surg Res. 2003; 110:332–337.
Article29. Renlund N, Pieretti-Vanmarcke R, O'Neill FH, Zhang L, Donahoe PK, Teixeira J. c-Jun N-terminal kinase inhibitor II (SP600125) activates Mullerian inhibiting substance type II receptor-mediated signal transduction. Endocrinology. 2008; 149:108–115.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mullerian inhibiting substance/anti-Mullerian hormone: A novel treatment for gynecologic tumors
- Antiproliferative Effects of Mullerian Inhibiting Substance on Human Ovarian Cancer Cell Lines
- Mullerian inhibiting substance as a predictive marker of menopausal transition
- Anti-Mullerian hormone and female reproduction
- Expression of mullerian inhibiting substance and its receptor in ovarian neoplsia