Obstet Gynecol Sci.
2014 Sep;57(5):343-357. 10.5468/ogs.2014.57.5.343.
Mullerian inhibiting substance/anti-Mullerian hormone: A novel treatment for gynecologic tumors
- Affiliations
-
- 1Department of Obstetrics and Gynecology, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 2Pediatric Surgical Research Laboratories, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA. pdonahoe@partners.org
- KMID: 2051715
- DOI: http://doi.org/10.5468/ogs.2014.57.5.343
Abstract
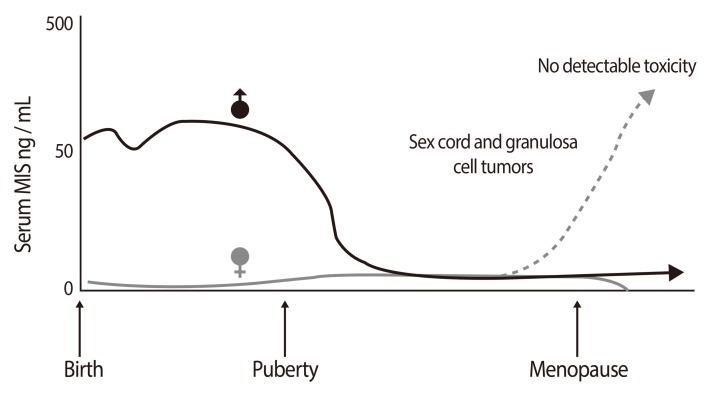
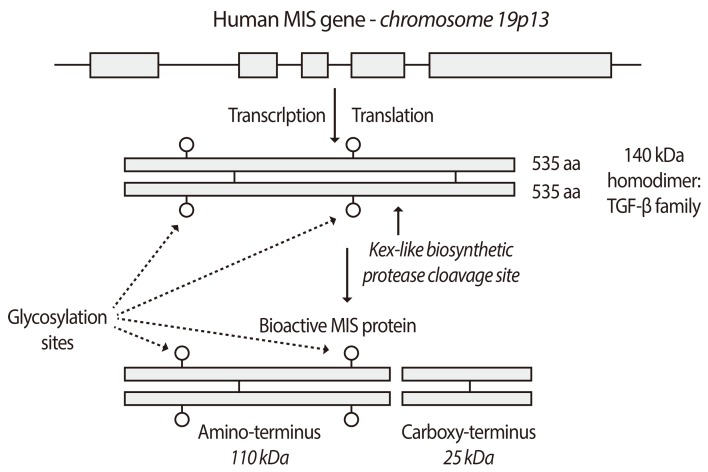
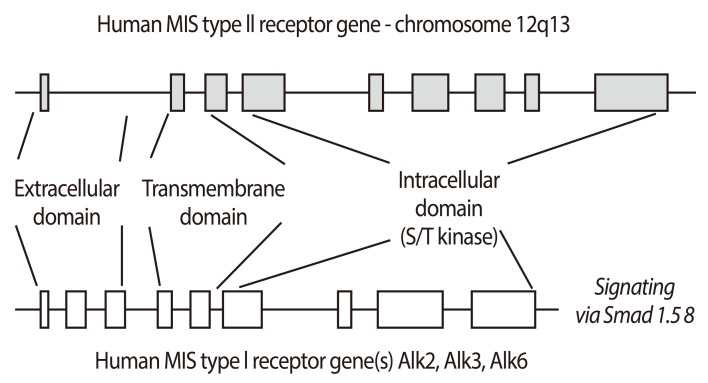
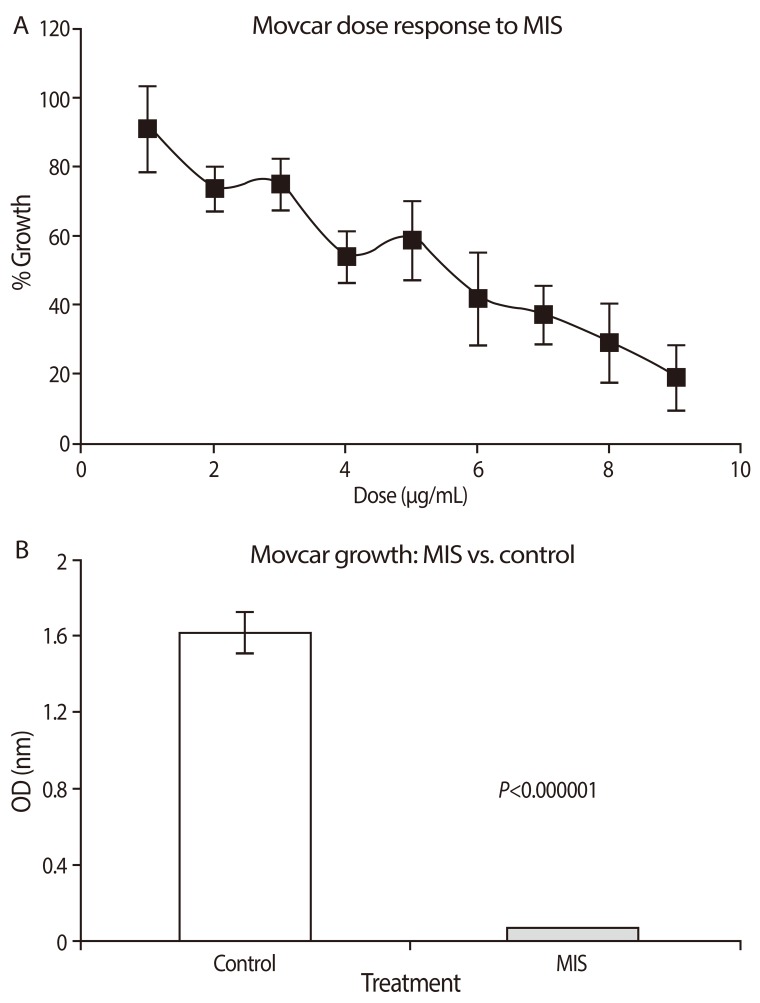
- Mullerian inhibiting substance (MIS), also called anti-Mullerian hormone (AMH), is a member of the transforming growth factor-beta super-family of growth and differentiation response modifiers. It is produced in immature Sertoli cells in male embryos and binds to MIS/AMH receptors in primordial Mullerian ducts to cause regression of female reproductive structures that are the precursors to the fallopian tubes, the surface epithelium of the ovaries, the uterus, the cervix, and the upper third of the vagina. Because most gynecologic tumors originate from Mullerian duct-derived tissues, and since MIS/AMH causes regression of the Mullerian duct in male embryos, it is expected to inhibit the growth of gynecologic tumors. Purified recombinant human MIS/AMH causes growth inhibition of epithelial ovarian cancer cells and cell lines in vitro and in vitro via MIS receptor-mediated mechanism. Furthermore, several lines of evidence suggest that MIS/AMH inhibits proliferation in tissues and cell lines of other MIS/AMH receptor-expressing gynecologic tumors such as cervical, endometrial, breast, and in endometriosis as well. These findings indicate that bioactive MIS/AMH recombinant protein should be tested in patients against tumors expressing the MIS/AMH receptor complex, perhaps beginning with ovarian cancer because it has the worst prognosis. The molecular tools to identify MIS/AMH receptor expressing ovarian and other cancers are in place, thus, it is possible to select patients for treatment. An MIS/AMH ELISA exists to follow administered doses of MIS/AMH, as well. Clinical trials await the production of sufficient supplies of qualified recombinant human MIS/AMH for this purpose.
Keyword
MeSH Terms
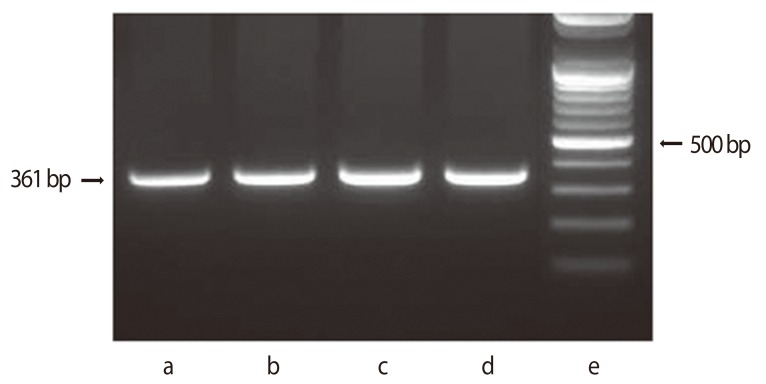
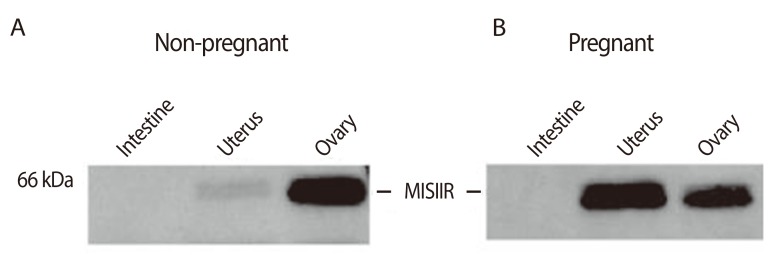
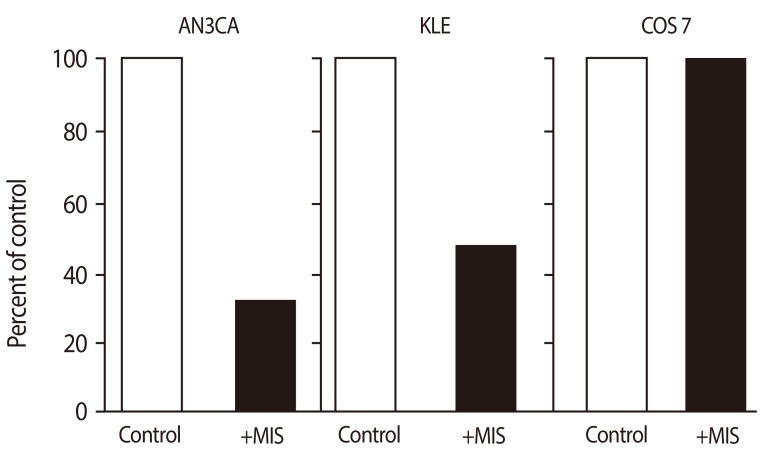
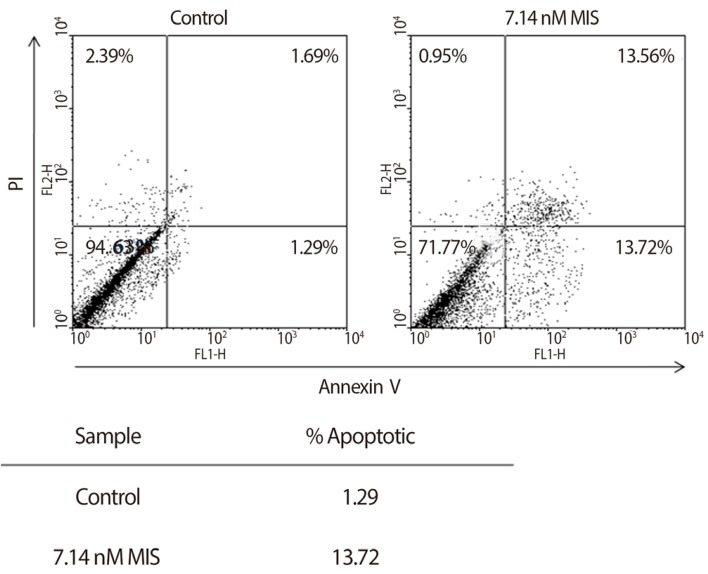
Figure
Cited by 1 articles
-
The expression of Müllerian inhibiting substance/anti-Müllerian hormone type II receptor in myoma and adenomyosis
Shin Young Kim, Hye Min Moon, Min Kyoung Lee, Youn Jee Chung, Jae Yen Song, Hyun Hee Cho, Mee Ran Kim, Jang Heub Kim
Obstet Gynecol Sci. 2018;61(1):127-134. doi: 10.5468/ogs.2018.61.1.127.
Reference
-
1. Goodfellow PN, Lovell-Badge R. SRY and sex determination in mammals. Annu Rev Genet. 1993; 27:71–92. PMID: 8122913.
Article2. Jost A. Recherches sur la differenciation sexuelle de l'embryon de lapin. Arch Anat Microsc Morphol Exp. 1947; 36:271–315.3. Jost A. Problems of fetal endocrinology: the gonadal and hypophyseal hormones. Rec Prog Horm Res. 1953; 8:379–418.4. Price JM, Donahoe PK, Ito Y, Hendren WH 3rd. Programmed cell death in the Mullerian duct induced by Mullerian inhibiting substance. Am J Anat. 1977; 149:353–375. PMID: 879051.5. Price JM, Donahoe PK, Ito Y. Involution of the female Mullerian duct of the fetal rat in the organ-culture assay for the detection of Mullerian Inhibiting Substance. Am J Anat. 1979; 156:265–284. PMID: 506954.
Article6. Hayashi A, Donahoe PK, Budzik GP, Trelstad RL. Periductal and matrix glycosaminoglycans in rat Mullerian duct development and regression. Dev Biol. 1982; 92:16–26. PMID: 7106377.
Article7. Trelstad RL, Hayashi A, Hayashi K, Donahoe PK. The epithelial-mesenchymal interface of the male rate Mullerian duct: loss of basement membrane integrity and ductal regression. Dev Biol. 1982; 92:27–40. PMID: 7106385.8. Ikawa H, Trelstad RL, Hutson JM, Manganaro TF, Donahoe PK. Changing patterns of fibronectin, laminin, type IV collagen, and a basement membrane proteoglycan during rat Mullerian duct regression. Dev Biol. 1984; 102:260–263. PMID: 6365654.
Article9. Dyche WJ. A comparative study of the differentiation and involution of the Mullerian duct and Wolffian duct in the male and female fetal mouse. J Morphol. 1979; 162:175–209. PMID: 537099.
Article10. Josso N. In vitro synthesis of mullerian-inhibiting hormone by seminiferous tubules isolated from the calf fetal testis. Endocrinology. 1973; 93:829–834. PMID: 4728217.
Article11. Blanchard MG, Josso N. Source of the anti-Mullerian hormone synthesized by the fetal testis: Mullerian-inhibiting activity of fetal bovine Sertoli cells in tissue culture. Pediatr Res. 1974; 8:968–971. PMID: 4444868.12. Picon R. Action of the fetal testis on the development in vitro of the Mullerian ducts in the rat. Arch Anat Microsc Morphol Exp. 1969; 58:1–19. PMID: 5822330.13. Josso N, Forest MG, Picard JY. Mullerian-inhibiting activity of calf fetal testes: relationship to testosterone and protein synthesis. Biol Reprod. 1975; 13:163–167. PMID: 1222192.14. Donahoe PK, Ito Y, Price JM, Hendren WH 3rd. Mullerian inhibiting substance activity in bovine fetal, newborn and prepubertal testes. Biol Reprod. 1977; 16:238–243. PMID: 556673.15. Josso N. Permeability of membranes to the Mullerian-inhibiting substance synthesized by the human fetal testis in vitro: a clue to its biochemical nature. J Clin Endocrinol Metab. 1972; 34:265–270. PMID: 5059759.16. Donahoe PK, Ito Y, Morikawa Y, Hendren WH. Mullerian inhibiting substance in human testes after birth. J Pediatr Surg. 1977; 12:323–330. PMID: 17665.
Article17. Shen WH, Moore CC, Ikeda Y, Parker KL, Ingraham HA. Nuclear receptor steroidogenic factor 1 regulates the mullerian inhibiting substance gene: a link to the sex determination cascade. Cell. 1994; 77:651–661. PMID: 8205615.18. Giuili G, Shen WH, Ingraham HA. The nuclear receptor SF-1 mediates sexually dimorphic expression of Mullerian Inhibiting Substance, in vivo. Development. 1997; 124:1799–1807. PMID: 9165127.
Article19. Arango NA, Lovell-Badge R, Behringer RR. Targeted mutagenesis of the endogenous mouse Mis gene promoter: in vivo definition of genetic pathways of vertebrate sexual development. Cell. 1999; 99:409–419. PMID: 10571183.20. Hossain A, Saunders GF. Role of Wilms tumor 1 (WT1) in the transcriptional regulation of the Mullerian-inhibiting substance promoter. Biol Reprod. 2003; 69:1808–1814. PMID: 12855602.21. Nachtigal MW, Hirokawa Y, Enyeart-VanHouten DL, Flanagan JN, Hammer GD, Ingraham HA. Wilms' tumor 1 and Dax-1 modulate the orphan nuclear receptor SF-1 in sex-specific gene expression. Cell. 1998; 93:445–454. PMID: 9590178.
Article22. Tremblay JJ, Viger RS. Nuclear receptor Dax-1 represses the transcriptional cooperation between GATA-4 and SF-1 in Sertoli cells. Biol Reprod. 2001; 64:1191–1199. PMID: 11259267.23. Lasala C, Carre-Eusebe D, Picard JY, Rey R. Subcellular and molecular mechanisms regulating anti-Mullerian hormone gene expression in mammalian and nonmammalian species. DNA Cell Biol. 2004; 23:572–585. PMID: 15383177.24. Teixeira J, Fynn-Thompson E, Payne AH, Donahoe PK. Mullerian-inhibiting substance regulates androgen synthesis at the transcriptional level. Endocrinology. 1999; 140:4732–4738. PMID: 10499532.25. Laurich VM, Trbovich AM, O'Neill FH, Houk CP, Sluss PM, Payne AH, et al. Mullerian inhibiting substance blocks the protein kinase A-induced expression of cytochrome p450 17alpha-hydroxylase/C(17-20) lyase mRNA in a mouse Leydig cell line independent of cAMP responsive element binding protein phosphorylation. Endocrinology. 2002; 143:3351–3360. PMID: 12193547.26. Sriraman V, Niu E, Matias JR, Donahoe PK, MacLaughlin DT, Hardy MP, et al. Mullerian inhibiting substance inhibits testosterone synthesis in adult rats. J Androl. 2001; 22:750–758. PMID: 11545286.27. Bezard J, Vigier B, Tran D, Mauleon P, Josso N. Immunocytochemical study of anti-Mullerian hormone in sheep ovarian follicles during fetal and post-natal development. J Reprod Fertil. 1987; 80:509–516. PMID: 3309279.
Article28. MacLaughlin DT, Donahoe PK. Mullerian inhibiting substance/anti-Mullerian hormone: a potential therapeutic agent for human ovarian and other cancers. Future Oncol. 2010; 6:391–405. PMID: 20222796.29. Hudson PL, Dougas I, Donahoe PK, Cate RL, Epstein J, Pepinsky RB, et al. An immunoassay to detect human mullerian inhibiting substance in males and females during normal development. J Clin Endocrinol Metab. 1990; 70:16–22. PMID: 2294129.
Article30. Josso N, Legeai L, Forest MG, Chaussain JL, Brauner R. An enzyme linked immunoassay for anti-mullerian hormone: a new tool for the evaluation of testicular function in infants and children. J Clin Endocrinol Metab. 1990; 70:23–27. PMID: 1688440.31. Baker ML, Metcalfe SA, Hutson JM. Serum levels of mullerian inhibiting substance in boys from birth to 18 years, as determined by enzyme immunoassay. J Clin Endocrinol Metab. 1990; 70:11–15. PMID: 2403569.
Article32. Ueno S, Manganaro TF, Donahoe PK. Human recombinant mullerian inhibiting substance inhibition of rat oocyte meiosis is reversed by epidermal growth factor in vitro. Endocrinology. 1988; 123:1652–1659. PMID: 2456917.33. Kim JH, Seibel MM, MacLaughlin DT, Donahoe PK, Ransil BJ, Hametz PA, et al. The inhibitory effects of mullerian-inhibiting substance on epidermal growth factor induced proliferation and progesterone production of human granulosa-luteal cells. J Clin Endocrinol Metab. 1992; 75:911–917. PMID: 1517385.
Article34. Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, et al. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology. 1999; 140:5789–5796. PMID: 10579345.35. Seifer DB, MacLaughlin DT, Penzias AS, Behrman HR, Asmundson L, Donahoe PK, et al. Gonadotropin-releasing hormone agonist-induced differences in granulosa cell cycle kinetics are associated with alterations in follicular fluid mullerian-inhibiting substance and androgen content. J Clin Endocrinol Metab. 1993; 76:711–714. PMID: 8445031.
Article36. Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum mullerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002; 77:468–471. PMID: 11872196.37. Trbovich AM, Sluss PM, Laurich VM, O'Neill FH, MacLaughlin DT, Donahoe PK, et al. Mullerian Inhibiting Substance lowers testosterone in luteinizing hormone-stimulated rodents. Proc Natl Acad Sci U S A. 2001; 98:3393–3397. PMID: 11248089.
Article38. Lee MM, Seah CC, Masiakos PT, Sottas CM, Preffer FI, Donahoe PK, et al. Mullerian-inhibiting substance type II receptor expression and function in purified rat Leydig cells. Endocrinology. 1999; 140:2819–2827. PMID: 10342873.
Article39. Baarends WM, Hoogerbrugge JW, Post M, Visser JA, De Rooij DG, Parvinen M, et al. Anti-mullerian hormone and anti-mullerian hormone type II receptor messenger ribonucleic acid expression during postnatal testis development and in the adult testis of the rat. Endocrinology. 1995; 136:5614–5622. PMID: 7588316.40. Bedecarrats GY, O'Neill FH, Norwitz ER, Kaiser UB, Teixeira J. Regulation of gonadotropin gene expression by Mullerian inhibiting substance. Proc Natl Acad Sci U S A. 2003; 100:9348–9353. PMID: 12878721.
Article41. Wang PY, Koishi K, McGeachie AB, Kimber M, Maclaughlin DT, Donahoe PK, et al. Mullerian inhibiting substance acts as a motor neuron survival factor in vitro. Proc Natl Acad Sci U S A. 2005; 102:16421–16425. PMID: 16260730.
Article42. Segev DL, Hoshiya Y, Hoshiya M, Tran TT, Carey JL, Stephen AE, et al. Mullerian-inhibiting substance regulates NF-kappa B signaling in the prostate in vitro and in vivo. Proc Natl Acad Sci U S A. 2002; 99:239–244. PMID: 11773638.43. Hoshiya Y, Gupta V, Segev DL, Hoshiya M, Carey JL, Sasur LM, et al. Mullerian Inhibiting Substance induces NFkB signaling in breast and prostate cancer cells. Mol Cell Endocrinol. 2003; 211:43–49. PMID: 14656475.
Article44. Segev DL, Hoshiya Y, Stephen AE, Hoshiya M, Tran TT, MacLaughlin DT, et al. Mullerian inhibiting substance regulates NFkappaB signaling and growth of mammary epithelial cells in vivo. J Biol Chem. 2001; 276:26799–26806. PMID: 11356848.45. Hoshiya Y, Gupta V, Kawakubo H, Brachtel E, Carey JL, Sasur L, et al. Mullerian inhibiting substance promotes interferon gamma-induced gene expression and apoptosis in breast cancer cells. J Biol Chem. 2003; 278:51703–51712. PMID: 14532292.46. Gupta V, Carey JL, Kawakubo H, Muzikansky A, Green JE, Donahoe PK, et al. Mullerian inhibiting substance suppresses tumor growth in the C3(1)T antigen transgenic mouse mammary carcinoma model. Proc Natl Acad Sci U S A. 2005; 102:3219–3224. PMID: 15728372.
Article47. Parry RL, Chin TW, Epstein J, Hudson PL, Powell DM, Donahoe PK. Recombinant human mullerian inhibiting substance inhibits human ocular melanoma cell lines in vitro and in vivo. Cancer Res. 1992; 52:1182–1186. PMID: 1531323.48. Vigier B, Picard JY, Josso N. A monoclonal antibody against bovine anti-Mullerian hormone. Endocrinology. 1982; 110:131–137. PMID: 6895620.49. Shima H, Donahoe PK, Budzik GP, Kamagata S, Hudson P, Mudgett-Hunter M. Production of monoclonal antibodies for affinity purification of bovine mullerian inhibiting substance activity. Hybridoma. 1984; 3:201–214. PMID: 6548727.
Article50. Picard JY, Josso N. Anti-Mullerian hormone: estimation of molecular weight by gel filtration. Biomedicine. 1976; 25:147–150. PMID: 986198.51. Picard JY, Josso N. Purification of testicular anti-Mullerian hormone allowing direct visualization of the pure glycoprotein and determination of yield and purification factor. Mol Cell Endocrinol. 1984; 34:23–29. PMID: 6546551.52. Swann DA, Donahoe PK, Ito Y, Morikawa Y, Hendren WH. Extraction of Mullerian inhibiting substance from newborn calf testis. Dev Biol. 1979; 69:73–84. PMID: 446900.
Article53. Budzik GP, Swann DA, Hayashi A, Donahoe PK. Enhanced purification of Mullerian inhibiting substance by lectin affinity chromatography. Cell. 1980; 21:909–915. PMID: 6893682.
Article54. Budzik GP, Powell SM, Kamagata S, Donahoe PK. Mullerian inhibiting substance fractionation by dye affinity chromatography. Cell. 1983; 34:307–314. PMID: 6411352.
Article55. Cate RL, Mattaliano RJ, Hession C, Tizard R, Farber NM, Cheung A, et al. Isolation of the bovine and human genes for Mullerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986; 45:685–698. PMID: 3754790.56. Picard JY, Benarous R, Guerrier D, Josso N, Kahn A. Cloning and expression of cDNA for anti-mullerian hormone. Proc Natl Acad Sci U S A. 1986; 83:5464–5468. PMID: 2426698.
Article57. Cohen-Haguenauer O, Picard JY, Mattei MG, Serero S, Nguyen VC, de Tand MF, et al. Mapping of the gene for anti-mullerian hormone to the short arm of human chromosome 19. Cytogenet Cell Genet. 1987; 44:2–6. PMID: 3028714.58. Pepinsky RB, Sinclair LK, Chow EP, Mattaliano RJ, Manganaro TF, Donahoe PK, et al. Proteolytic processing of mullerian inhibiting substance produces a transforming growth factor-beta-like fragment. J Biol Chem. 1988; 263:18961–18964. PMID: 2974034.
Article59. MacLaughlin DT, Hudson PL, Graciano AL, Kenneally MK, Ragin RC, Manganaro TF, et al. Mullerian duct regression and antiproliferative bioactivities of mullerian inhibiting substance reside in its carboxy-terminal domain. Endocrinology. 1992; 131:291–296. PMID: 1612008.
Article60. Wilson CA, di Clemente N, Ehrenfels C, Pepinsky RB, Josso N, Vigier B, et al. Mullerian inhibiting substance requires its N-terminal domain for maintenance of biological activity, a novel finding within the transforming growth factor-beta superfamily. Mol Endocrinol. 1993; 7:247–257. PMID: 8469238.
Article61. Ragin RC, Donahoe PK, Kenneally MK, Ahmad MF, MacLaughlin DT. Human mullerian inhibiting substance: enhanced purification imparts biochemical stability and restores antiproliferative effects. Protein Expr Purif. 1992; 3:236–245. PMID: 1392620.62. Lorenzo HK, Teixeira J, Pahlavan N, Laurich VM, Donahoe PK, MacLaughlin DT. New approaches for high-yield purification of Mullerian inhibiting substance improve its bioactivity. J Chromatogr B Analyt Technol Biomed Life Sci. 2002; 766:89–98.63. Baarends WM, van Helmond MJ, Post M, van der Schoot PJ, Hoogerbrugge JW, de Winter JP, et al. A novel member of the transmembrane serine/threonine kinase receptor family is specifically expressed in the gonads and in mesenchymal cells adjacent to the mullerian duct. Development. 1994; 120:189–197. PMID: 8119126.
Article64. Di Clemente N, Wilson C, Faure E, Boussin L, Carmillo P, Tizard R, et al. Cloning, expression, and alternative splicing of the receptor for anti-Mullerian hormone. Mol Endocrinol. 1994; 8:1006–1020. PMID: 7997230.65. Teixeira J, He WW, Shah PC, Morikawa N, Lee MM, Catlin EA, et al. Developmental expression of a candidate mullerian inhibiting substance type II receptor. Endocrinology. 1996; 137:160–165. PMID: 8536608.66. Mishina Y, Tizard R, Deng JM, Pathak BG, Copeland NG, Jenkins NA, et al. Sequence, genomic organization, and chromosomal location of the mouse Mullerian-inhibiting substance type II receptor gene. Biochem Biophys Res Commun. 1997; 237:741–746. PMID: 9299437.67. Imbeaud S, Faure E, Lamarre I, Mattei MG, di Clemente N, Tizard R, et al. Insensitivity to anti-mullerian hormone due to a mutation in the human anti-mullerian hormone receptor. Nat Genet. 1995; 11:382–388. PMID: 7493017.68. Racine C, Rey R, Forest MG, Louis F, Ferre A, Huhtaniemi I, et al. Receptors for anti-mullerian hormone on Leydig cells are responsible for its effects on steroidogenesis and cell differentiation. Proc Natl Acad Sci U S A. 1998; 95:594–599. PMID: 9435237.
Article69. Baarends WM, Uilenbroek JT, Kramer P, Hoogerbrugge JW, van Leeuwen EC, Themmen AP, et al. Anti-mullerian hormone and anti-mullerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology. 1995; 136:4951–4962. PMID: 7588229.70. Renaud EJ, MacLaughlin DT, Oliva E, Rueda BR, Donahoe PK. Endometrial cancer is a receptor-mediated target for Mullerian Inhibiting Substance. Proc Natl Acad Sci U S A. 2005; 102:111–116. PMID: 15618407.
Article71. Josso N, Belville C, di Clemente N, Picard JY. AMH and AMH receptor defects in persistent Mullerian duct syndrome. Hum Reprod Update. 2005; 11:351–356. PMID: 15878900.72. Hoshiya M, Christian BP, Cromie WJ, Kim H, Zhan Y, MacLaughlin DT, et al. Persistent Mullerian duct syndrome caused by both a 27-bp deletion and a novel splice mutation in the MIS type II receptor gene. Birth Defects Res A Clin Mol Teratol. 2003; 67:868–874. PMID: 14745940.
Article73. Gouedard L, Chen YG, Thevenet L, Racine C, Borie S, Lamarre I, et al. Engagement of bone morphogenetic protein type IB receptor and Smad1 signaling by anti-Mullerian hormone and its type II receptor. J Biol Chem. 2000; 275:27973–27978. PMID: 10854429.74. Visser JA, Olaso R, Verhoef-Post M, Kramer P, Themmen AP, Ingraham HA. The serine/threonine transmembrane receptor ALK2 mediates Mullerian inhibiting substance signaling. Mol Endocrinol. 2001; 15:936–945. PMID: 11376112.
Article75. Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat Genet. 2002; 32:408–410. PMID: 12368913.76. He WW, Gustafson ML, Hirobe S, Donahoe PK. Developmental expression of four novel serine/threonine kinase receptors homologous to the activin/transforming growth factor-beta type II receptor family. Dev Dyn. 1993; 196:133–142. PMID: 8395914.77. Clarke TR, Hoshiya Y, Yi SE, Liu X, Lyons KM, Donahoe PK. Mullerian inhibiting substance signaling uses a bone morphogenetic protein (BMP)-like pathway mediated by ALK2 and induces SMAD6 expression. Mol Endocrinol. 2001; 15:946–959. PMID: 11376113.
Article78. Gu Z, Reynolds EM, Song J, Lei H, Feijen A, Yu L, et al. The type I serine/threonine kinase receptor ActRIA (ALK2) is required for gastrulation of the mouse embryo. Development. 1999; 126:2551–2561. PMID: 10226013.
Article79. Lee MM, Donahoe PK, Silverman BL, Hasegawa T, Hasegawa Y, Gustafson ML, et al. Measurements of serum mullerian inhibiting substance in the evaluation of children with nonpalpable gonads. N Engl J Med. 1997; 336:1480–1486. PMID: 9154766.
Article80. Van Rooij IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, de Jong FH, et al. Serum anti-Mullerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002; 17:3065–3071. PMID: 12456604.
Article81. Muttukrishna S, Suharjono H, McGarrigle H, Sathanandan M. Inhibin B and anti-Mullerian hormone: markers of ovarian response in IVF/ICSI patients? BJOG. 2004; 111:1248–1253. PMID: 15521870.
Article82. Hazout A, Bouchard P, Seifer DB, Aussage P, Junca AM, Cohen-Bacrie P. Serum antimullerian hormone/mullerian-inhibiting substance appears to be a more discriminatory marker of assisted reproductive technology outcome than follicle-stimulating hormone, inhibin B, or estradiol. Fertil Steril. 2004; 82:1323–1329. PMID: 15533354.83. Penarrubia J, Fabregues F, Manau D, Creus M, Casals G, Casamitjana R, et al. Basal and stimulation day 5 anti-Mullerian hormone serum concentrations as predictors of ovarian response and pregnancy in assisted reproductive technology cycles stimulated with gonadotropin-releasing hormone agonist: gonadotropin treatment. Hum Reprod. 2005; 20:915–922. PMID: 15665015.84. Eldar-Geva T, Ben-Chetrit A, Spitz IM, Rabinowitz R, Markowitz E, Mimoni T, et al. Dynamic assays of inhibin B, anti-Mullerian hormone and estradiol following FSH stimulation and ovarian ultrasonography as predictors of IVF outcome. Hum Reprod. 2005; 20:3178–3183. PMID: 16113044.
Article85. Silberstein T, MacLaughlin DT, Shai I, Trimarchi JR, Lambert-Messerlian G, Seifer DB, et al. Mullerian inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006; 21:159–163. PMID: 16123085.86. Gustafson ML, Lee MM, Scully RE, Moncure AC, Hirakawa T, Goodman A, et al. Mullerian inhibiting substance as a marker for ovarian sex-cord tumor. N Engl J Med. 1992; 326:466–471. PMID: 1732773.87. Gustafson ML, Lee MM, Asmundson L, MacLaughlin DT, Donahoe PK. Mullerian inhibiting substance in the diagnosis and management of intersex and gonadal abnormalities. J Pediatr Surg. 1993; 28:439–444. PMID: 8468660.88. Chang HL, Pahlavan N, Halpern EF, MacLaughlin DT. Serum Mullerian Inhibiting Substance/anti-Mullerian hormone levels in patients with adult granulosa cell tumors directly correlate with aggregate tumor mass as determined by pathology or radiology. Gynecol Oncol. 2009; 114:57–60. PMID: 19359032.89. Dutertre M, Gouedard L, Xavier F, Long WQ, di Clemente N, Picard JY, et al. Ovarian granulosa cell tumors express a functional membrane receptor for anti-Mullerian hormone in transgenic mice. Endocrinology. 2001; 142:4040–4046. PMID: 11517183.
Article90. Donahoe PK, Clarke T, Teixeira J, Maheswaran S, MacLaughlin DT. Enhanced purification and production of Mullerian inhibiting substance for therapeutic applications. Mol Cell Endocrinol. 2003; 211:37–42. PMID: 14656474.91. Teixeira J, Maheswaran S, Donahoe PK. Mullerian inhibiting substance: an instructive developmental hormone with diagnostic and possible therapeutic applications. Endocr Rev. 2001; 22:657–674. PMID: 11588147.
Article92. Di Clemente N, Josso N, Gouedard L, Belville C. Components of the anti-Mullerian hormone signaling pathway in gonads. Mol Cell Endocrinol. 2003; 211:9–14. PMID: 14656470.93. Josso N, Clemente Nd. Transduction pathway of anti-Mullerian hormone, a sex-specific member of the TGF-beta family. Trends Endocrinol Metab. 2003; 14:91–97. PMID: 12591180.95. Donahoe PK, Swann DA, Hayashi A, Sullivan MD. Mullerian duct regression in the embryo correlated with cytotoxic activity against human ovarian cancer. Science. 1979; 205:913–915. PMID: 472712.96. Fuller AF Jr, Guy S, Budzik GP, Donahoe PK. Mullerian inhibiting substance inhibits colony growth of a human ovarian carcinoma cell line. J Clin Endocrinol Metab. 1982; 54:1051–1055. PMID: 6895900.
Article97. Masiakos PT, MacLaughlin DT, Maheswaran S, Teixeira J, Fuller AF Jr, Shah PC, et al. Human ovarian cancer, cell lines, and primary ascites cells express the human Mullerian inhibiting substance (MIS) type II receptor, bind, and are responsive to MIS. Clin Cancer Res. 1999; 5:3488–3499. PMID: 10589763.98. Stephen AE, Pearsall LA, Christian BP, Donahoe PK, Vacanti JP, MacLaughlin DT. Highly purified mullerian inhibiting substance inhibits human ovarian cancer in vivo. Clin Cancer Res. 2002; 8:2640–2646. PMID: 12171896.99. Berkenblit A, Cannistra SA. Advances in the management of epithelial ovarian cancer. J Reprod Med. 2005; 50:426–438. PMID: 16050567.100. Barbie TU, Barbie DA, MacLaughlin DT, Maheswaran S, Donahoe PK. Mullerian Inhibiting Substance inhibits cervical cancer cell growth via a pathway involving p130 and p107. Proc Natl Acad Sci U S A. 2003; 100:15601–15606. PMID: 14671316.
Article101. Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003; 53:5–26. PMID: 12568441.
Article102. Salom E, Almeida Z, Mirhashemi R. Management of recurrent ovarian cancer: evidence-based decisions. Curr Opin Oncol. 2002; 14:519–527. PMID: 12192271.
Article103. Donahoe PK, Fuller AF Jr, Scully RE, Guy SR, Budzik GP. Mullerian inhibiting substance inhibits growth of a human ovarian cancer in nude mice. Ann Surg. 1981; 194:472–480. PMID: 6895157.
Article104. Chin TW, Parry RL, Donahoe PK. Human mullerian inhibiting substance inhibits tumor growth in vitro and in vivo. Cancer Res. 1991; 51:2101–2106. PMID: 2009529.105. Ha TU, Segev DL, Barbie D, Masiakos PT, Tran TT, Dombkowski D, et al. Mullerian inhibiting substance inhibits ovarian cell growth through an Rb-independent mechanism. J Biol Chem. 2000; 275:37101–37109. PMID: 10958795.106. Fuller AF Jr, Krane IM, Budzik GP, Donahoe PK. Mullerian inhibiting substance reduction of colony growth of human gynecologic cancers in a stem cell assay. Gynecol Oncol. 1985; 22:135–148. PMID: 3932140.
Article107. Bakkum-Gamez JN, Aletti G, Lewis KA, Keeney GL, Thomas BM, Navarro-Teulon I, et al. Mullerian inhibiting substance type II receptor (MISIIR): a novel, tissue-specific target expressed by gynecologic cancers. Gynecol Oncol. 2008; 108:141–148. PMID: 17988723.108. Song JY, Chen KY, Kim SY, Kim MR, Ryu KS, Cha JH, et al. The expression of Mullerian inhibiting substance/anti-Mullerian hormone type II receptor protein and mRNA in benign, borderline and malignant ovarian neoplasia. Int J Oncol. 2009; 34:1583–1591. PMID: 19424576.
Article109. Connolly DC, Bao R, Nikitin AY, Stephens KC, Poole TW, Hua X, et al. Female mice chimeric for expression of the simian virus 40 TAg under control of the MISIIR promoter develop epithelial ovarian cancer. Cancer Res. 2003; 63:1389–1397. PMID: 12649204.110. Pieretti-Vanmarcke R, Donahoe PK, Szotek P, Manganaro T, Lorenzen MK, Lorenzen J, et al. Recombinant human Mullerian inhibiting substance inhibits long-term growth of MIS type II receptor-directed transgenic mouse ovarian cancers in vivo. Clin Cancer Res. 2006; 12:1593–1598. PMID: 16533786.111. MacLaughlin DT, Donahoe PK. Mullerian inhibiting substance: a potential nontoxic, naturally occurring therapeutic agent for certain human cancers. In : Jakowlew SB, editor. Transforming growth factor-[beta] in cancer therapy. 1st ed. Totowa: Humana Press;2008. p. 333–354.112. Nam SW, Jo YS, Eun JW, Song JY, Ryu KS, Lee JY, et al. Identification of large-scale characteristic genes of Mullerian inhibiting substance in human ovarian cancer cells. Int J Mol Med. 2009; 23:589–596. PMID: 19360316.
Article113. Pieretti-Vanmarcke R, Donahoe PK, Pearsall LA, Dinulescu DM, Connolly DC, Halpern EF, et al. Mullerian Inhibiting Substance enhances subclinical doses of chemotherapeutic agents to inhibit human and mouse ovarian cancer. Proc Natl Acad Sci U S A. 2006; 103:17426–17431. PMID: 17088539.
Article114. Song JY, Jo HH, Kim MR, Lew YO, Ryu KS, Cha JH, et al. Expression of Mullerian inhibiting substance type II receptor and antiproliferative effects of MIS on human cervical cancer. Int J Oncol. 2012; 40:2013–2021. PMID: 22344630.115. Hwang SJ, Suh MJ, Yoon JH, Kim MR, Ryu KS, Nam SW, et al. Identification of characteristic molecular signature of Mullerian inhibiting substance in human HPV-related cervical cancer cells. Int J Oncol. 2011; 39:811–820. PMID: 21573503.116. Sovak MA, Bellas RE, Kim DW, Zanieski GJ, Rogers AE, Traish AM, et al. Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. J Clin Invest. 1997; 100:2952–2960. PMID: 9399940.
Article117. Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ Jr, Sledge GW Jr. Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol. 1997; 17:3629–3639. PMID: 9199297.
Article118. Namkung J, Song JY, Jo HH, Kim MR, Lew YO, Donahoe PK, et al. Mullerian inhibiting substance induces apoptosis of human endometrial stromal cells in endometriosis. J Clin Endocrinol Metab. 2012; 97:3224–3230. PMID: 22761458.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Persistent Mullerian Duct Syndrome in a Boy with Transverse Testicular Ectopia: a Case Report
- The Author Response: Serum anti-Mullerian hormone levels and phenotypes of polycystic ovary syndrome
- Letter to the Editor: Serum anti-Mullerian hormone levels and phenotypes of polycystic ovary syndrome
- A Case of Persistent Mullerian Duct Syndrome
- Mullerian inhibiting substance as a predictive marker of menopausal transition