Cancer Res Treat.
2019 Apr;51(2):493-501. 10.4143/crt.2018.125.
EGFR Mutation Is Associated with Short Progression-Free Survival in Patients with Stage III Non-squamous Cell Lung Cancer Treated with Concurrent Chemoradiotherapy
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Medicine, Chung-Ang University College of Medicine, Seoul, Korea.
- 2Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. silk.ahn@samsung.com
- 4Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2464396
- DOI: http://doi.org/10.4143/crt.2018.125
Abstract
- PURPOSE
This study was conducted to evaluate the relationship between epidermal growth factor receptor (EGFR) mutation and clinical outcomes in patients with stage III non-squamous cell lung cancer treated with definitive concurrent chemoradiotherapy (CCRT).
MATERIALS AND METHODS
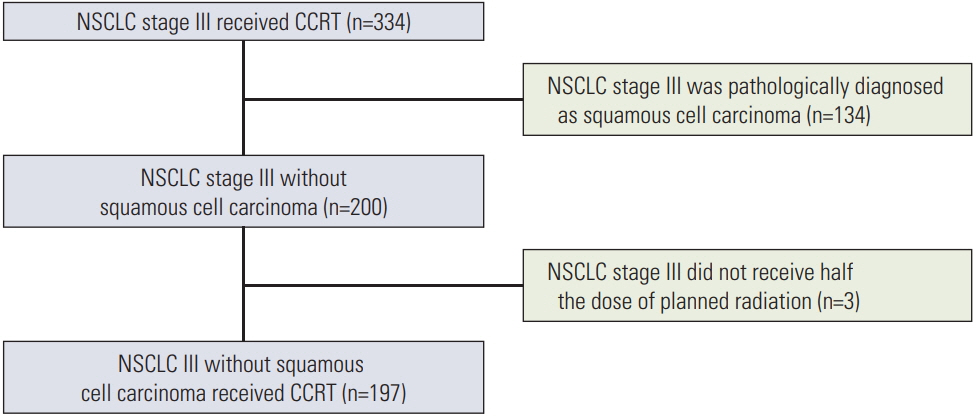
From January 2008 to December 2013, the medical records of 197 patients with stage III non- squamous non-small cell lung cancer treated with definitive CCRT were analyzed to determine progression-free survival (PFS) and overall survival (OS) according to EGFR mutation status.
RESULTS
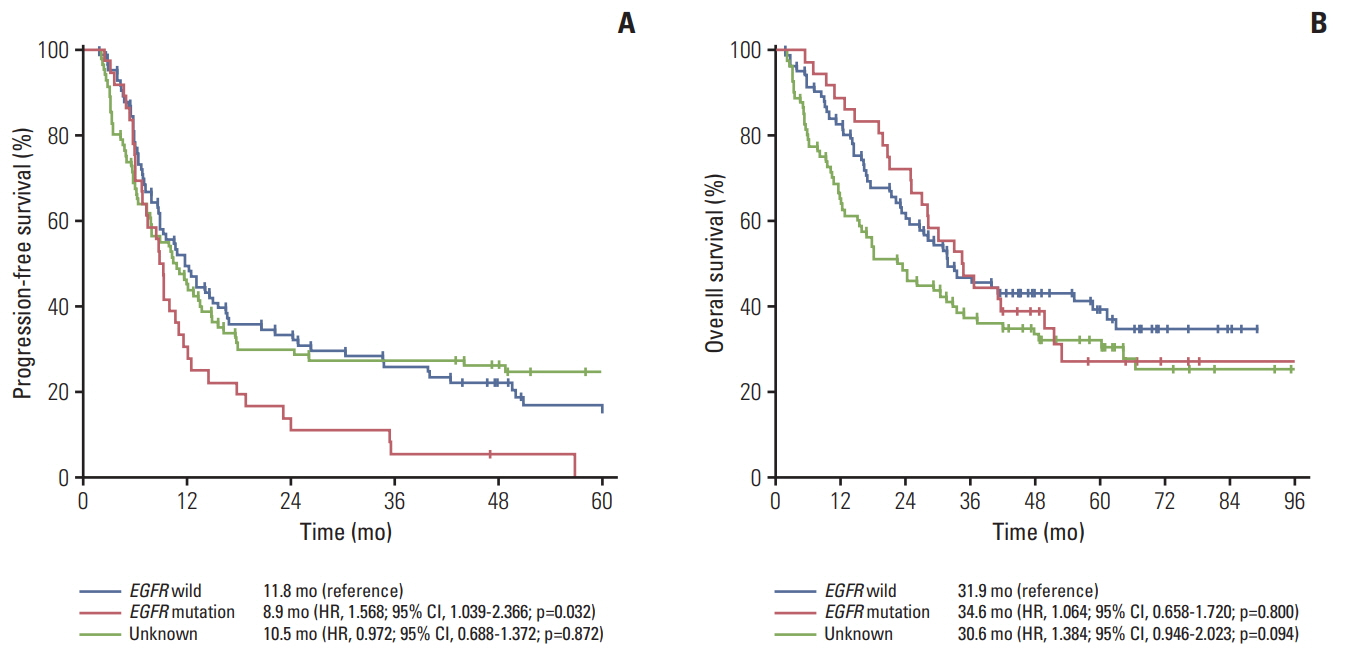
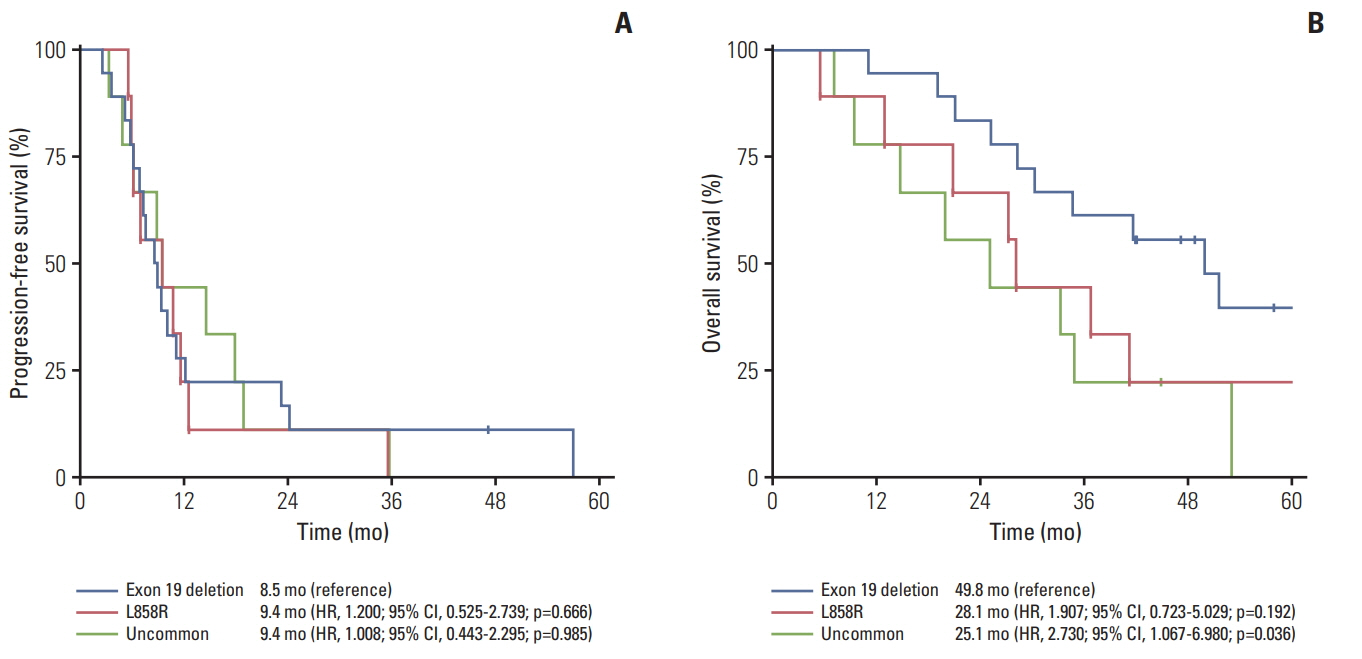
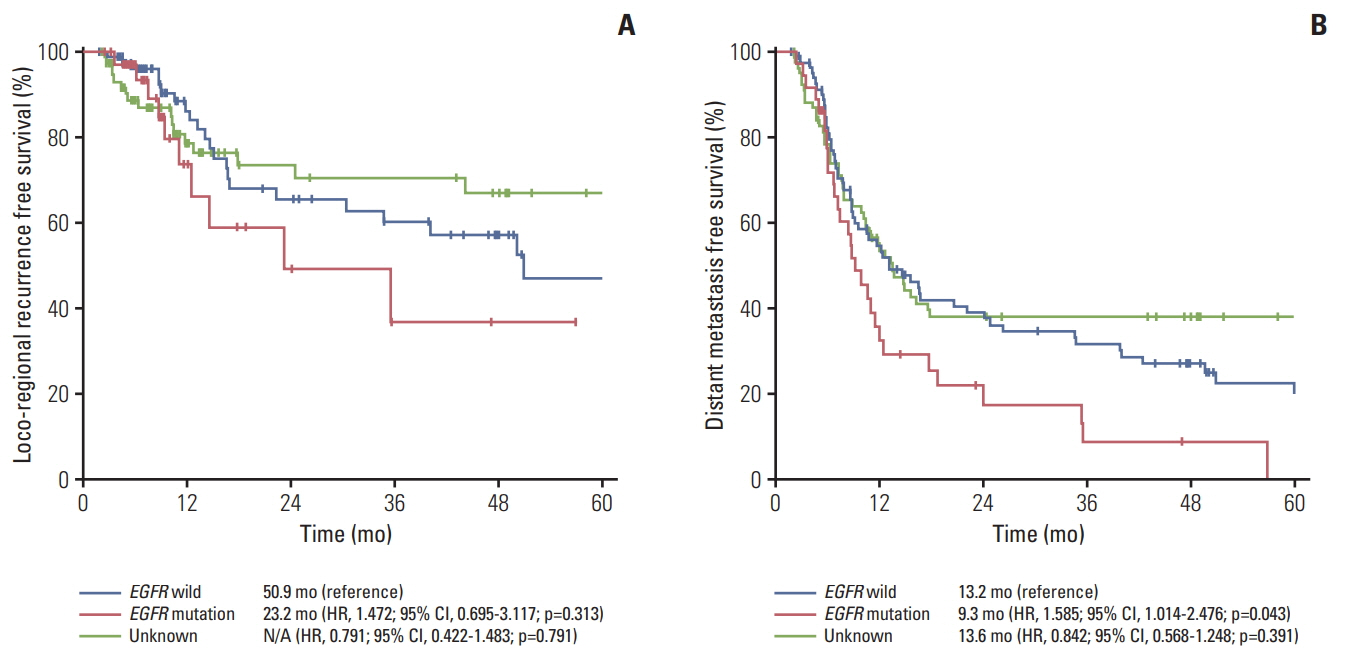
Among 197 eligible patients, 81 patients were EGFR wild type, 36 patients had an EGFR mutation (exon 19 Del, n=18; L858R, n=9, uncommon [G719X, L868, T790M], n=9), and 80 patients had unknown EGFR status. The median age was 59 years (range, 28 to 80 years) and 136 patients (69.0%) were male. The median follow-up duration was 66.5 months (range, 1.9 to 114.5 months). One hundred sixty-four patients (83.2%) experienced disease progression. Median PFS was 8.9 months for the EGFR mutation group, 11.8 months for EGFR wild type, and 10.5 months for the unknown EGFR group (p=0.013 and p=0.042, respectively). The most common site of metastasis in the EGFR mutant group was the brain. However, there was no significant difference in OS among the three groups (34.6 months for EGFR mutant group vs. 31.9 months for EGFR wild type vs. 22.6 months for EGFR unknown group; p=0.792 and p=0.284). A total of 29 patients (80.6%) with EGFR mutation were treated with EGFR tyrosine kinase inhibitor (gefitinib, n=24; erlotinib, n=3; afatinib, n=2) upon progression.
CONCLUSION
EGFR mutation is associatedwith short PFS and the brain is the most common site of distant metastasis in patients with stage III non- squamous cell lung cancer treated with CCRT.
Keyword
MeSH Terms
-
Brain
Carcinoma, Non-Small-Cell Lung
Chemoradiotherapy*
Disease Progression
Disease-Free Survival*
Epithelial Cells
Erlotinib Hydrochloride
Follow-Up Studies
Humans
Lung Neoplasms*
Lung*
Male
Medical Records
Neoplasm Metastasis
Protein-Tyrosine Kinases
Receptor, Epidermal Growth Factor
Erlotinib Hydrochloride
Protein-Tyrosine Kinases
Receptor, Epidermal Growth Factor
Figure
Cited by 1 articles
-
EGFR Mutation–Positive Unresectable Stage III Non-Squamous Lung Cancer Is Associated with a High Incidence of Brain Metastasis
Hongsik Kim, Sehhoon Park, Hyun Ae Jung, Jong-Mu Sun, Se-Hoon Lee, Jin Seok Ahn, Myung-Ju Ahn
Cancer Res Treat. 2023;55(2):498-505. doi: 10.4143/crt.2022.388.
Reference
-
References
1. Dillman RO, Seagren SL, Propert KJ, Guerra J, Eaton WL, Perry MC, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med. 1990; 323:940–5.
Article2. Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999; 17:2692–9.
Article3. Segawa Y, Kiura K, Takigawa N, Kamei H, Harita S, Hiraki S, et al. Phase III trial comparing docetaxel and cisplatin combination chemotherapy with mitomycin, vindesine, and cisplatin combination chemotherapy with concurrent thoracic radiotherapy in locally advanced non-small-cell lung cancer: OLCSG 0007. J Clin Oncol. 2010; 28:3299–306.
Article4. Yamamoto N, Nakagawa K, Nishimura Y, Tsujino K, Satouchi M, Kudo S, et al. Phase III study comparing second- and third-generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non-small-cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105. J Clin Oncol. 2010; 28:3739–45.
Article5. Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014; 311:1998–2006.
Article6. Yatabe Y, Kerr KM, Utomo A, Rajadurai P, Tran VK, Du X, et al. EGFR mutation testing practices within the Asia Pacific region: results of a multicenter diagnostic survey. J Thorac Oncol. 2015; 10:438–45.
Article7. Han JY, Kim SH, Lee YS, Lee SY, Hwang JA, Kim JY, et al. Comparison of targeted next-generation sequencing with conventional sequencing for predicting the responsiveness to epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) therapy in never-smokers with lung adenocarcinoma. Lung Cancer. 2014; 85:161–7.
Article8. Nguyen KS, Neal JW. First-line treatment of EGFR-mutant non-small-cell lung cancer: the role of erlotinib and other tyrosine kinase inhibitors. Biologics. 2012; 6:337–45.9. Li F, Bai H, Li X, Wu M, Yu R, Shi A, et al. Role of EGFR mutation status in patients with stage III non-squamous non-small cell lung cancer treated with chemoradiotherapy. Zhongguo Fei Ai Za Zhi. 2011; 14:715–8.10. Mak RH, Doran E, Muzikansky A, Kang J, Neal JW, Baldini EH, et al. Outcomes after combined modality therapy for EGFR-mutant and wild-type locally advanced NSCLC. Oncologist. 2011; 16:886–95.
Article11. Tanaka T, Nagai Y, Miyazawa H, Koyama N, Matsuoka S, Sutani A, et al. Reliability of the peptide nucleic acid-locked nucleic acid polymerase chain reaction clamp-based test for epidermal growth factor receptor mutations integrated into the clinical practice for non-small cell lung cancers. Cancer Sci. 2007; 98:246–52.
Article12. Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015; 16:187–99.
Article13. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
Article14. Ahn HK, Choi YL, Han JH, Ahn YC, Kim K, Kim J, et al. Epidermal growth factor receptor mutation and treatment outcome of mediastinoscopic N2 positive non-small cell lung cancer patients treated with neoadjuvant chemoradiotherapy followed by surgery. Lung Cancer. 2013; 79:300–6.
Article15. Kelly K, Chansky K, Gaspar LE, Albain KS, Jett J, Ung YC, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol. 2008; 26:2450–6.
Article16. Bhutani M, Pathak AK, Mao L. SWOG S0023: what meets the eye may be only half the truth. J Clin Oncol. 2008; 26:4848–9.
Article17. Tanaka K, Hida T, Oya Y, Oguri T, Yoshida T, Shimizu J, et al. EGFR mutation impact on definitive concurrent chemoradiation therapy for inoperable stage III adenocarcinoma. J Thorac Oncol. 2015; 10:1720–5.
Article18. Akamatsu H, Kaira K, Murakami H, Serizawa M, Koh Y, Ono A, et al. The impact of clinical outcomes according to EGFR mutation status in patients with locally advanced lung adenocarcinoma who recieved concurrent chemoradiotherapy. Am J Clin Oncol. 2014; 37:144–7.
Article19. Gore EM, Bae K, Wong SJ, Sun A, Bonner JA, Schild SE, et al. Phase III comparison of prophylactic cranial irradiation versus observation in patients with locally advanced non-small-cell lung cancer: primary analysis of radiation therapy oncology group study RTOG 0214. J Clin Oncol. 2011; 29:272–8.
Article20. Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017; 376:629–40.
Article21. Ballard P, Yates JW, Yang Z, Kim DW, Yang JC, Cantarini M, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016; 22:5130–40.
Article22. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017; 377:1919–29.
Article23. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015; 373:1627–39.
Article24. Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016; 387:1540–50.
Article25. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017; 389:255–65.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- EGFR Mutation–Positive Unresectable Stage III Non-Squamous Lung Cancer Is Associated with a High Incidence of Brain Metastasis
- Incorporating Erlotinib or Irinotecan Plus Cisplatin into Chemoradiotherapy for Stage III Non-small Cell Lung Cancer According to EGFR Mutation Status
- Does the efficacy of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor differ according to the type of EGFR mutation in non-small cell lung cancer?
- Lack of the Difference in Outcome between N2 and N3 Non-Small Cell Lung Cancer Patients Treated with Concurrent Chemoradiotherapy; A Retrospective Analysis of 186 Patients
- Prognostic significance of neutropenia during adjuvant concurrent chemoradiotherapy in early cervical cancer