J Periodontal Implant Sci.
2019 Oct;49(5):270-286. 10.5051/jpis.2019.49.5.270.
Vitamin D maintains E-cadherin intercellular junctions by downregulating MMP-9 production in human gingival keratinocytes treated by TNF-α
- Affiliations
-
- 1Laboratory for the Study of Molecular Biointerfaces, Department of Oral Histology and Developmental Biology, Program of Cell and Developmental Biology, School of Dentistry and Dental Research Institute, Seoul National University, Seoul, Korea. hyunmkim@snu.ac.kr
- KMID: 2461018
- DOI: http://doi.org/10.5051/jpis.2019.49.5.270
Abstract
- PURPOSE
Despite the well-known anti-inflammatory effects of vitamin D in periodontal health, its mechanism has not been fully elucidated. In the present study, the effect of vitamin D on strengthening E-cadherin junctions (ECJs) was explored in human gingival keratinocytes (HGKs). ECJs are the major type of intercellular junction within the junctional epithelium, where loose intercellular junctions develop and microbial invasion primarily occurs.
METHODS
HOK-16B cells, an immortalized normal human gingival cell line, were used for the study. To mimic the inflammatory environment, cells were treated with tumor necrosis factor-alpha (TNF-α). Matrix metalloproteinases (MMPs) in the culture medium were assessed by an MMP antibody microarray and gelatin zymography. The expression of various molecules was investigated using western blotting. The extent of ECJ development was evaluated by comparing the average relative extent of the ECJs around the periphery of each cell after immunocytochemical E-cadherin staining. Vitamin D receptor (VDR) expression was examined via immunohistochemical analysis.
RESULTS
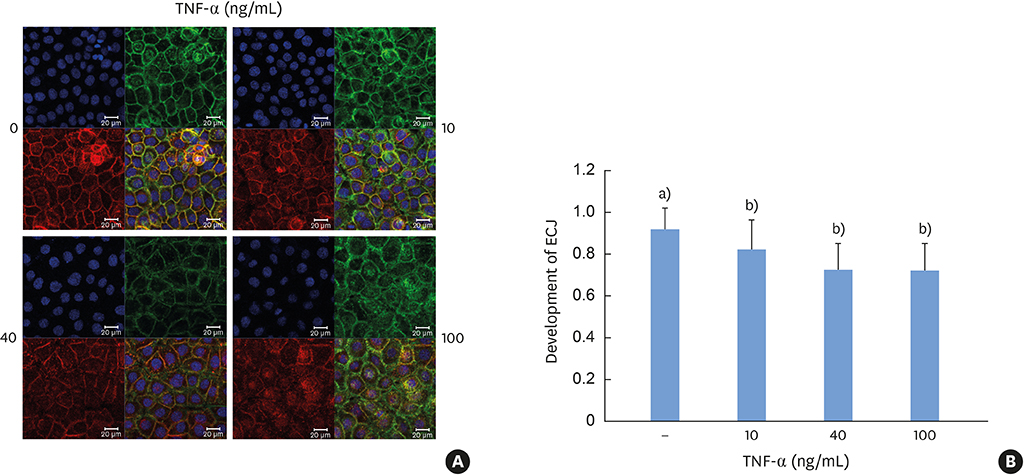
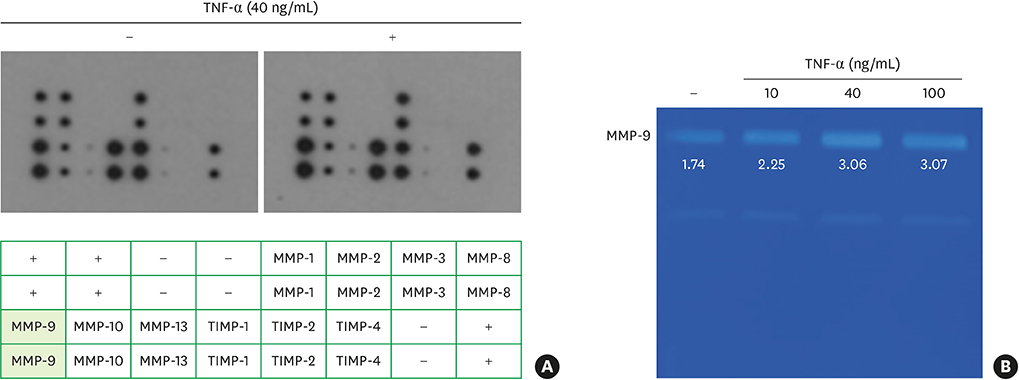
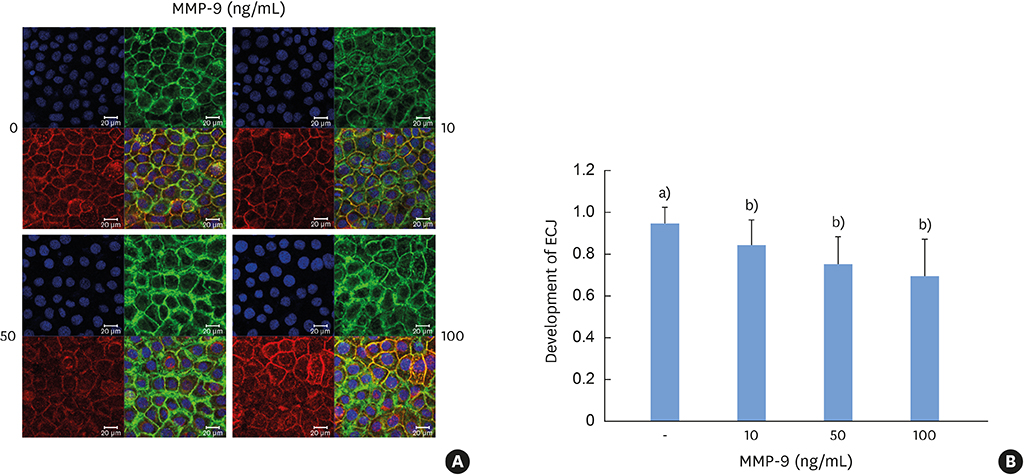
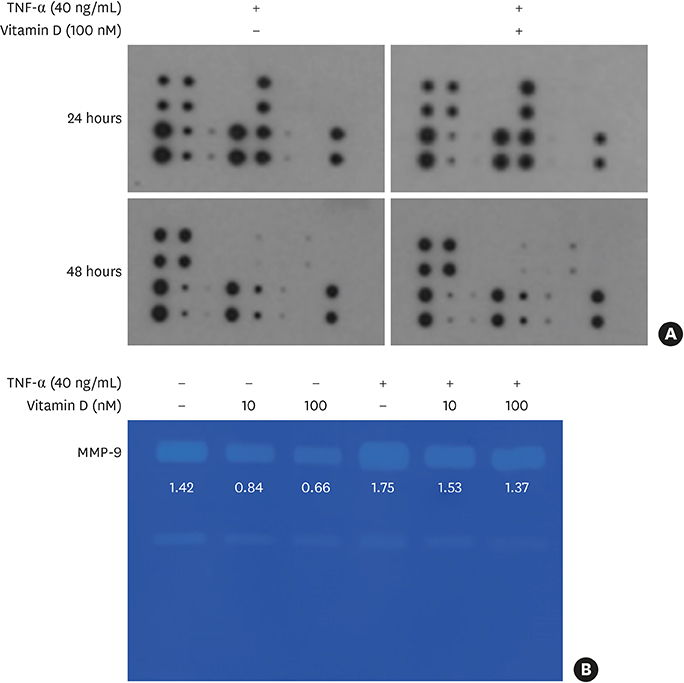
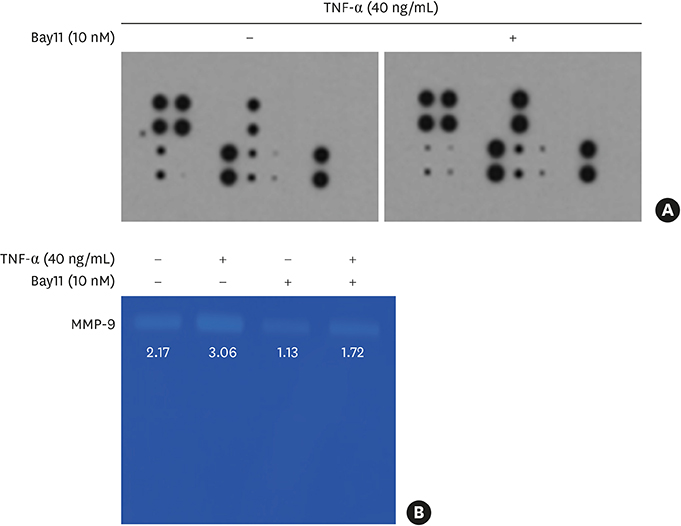
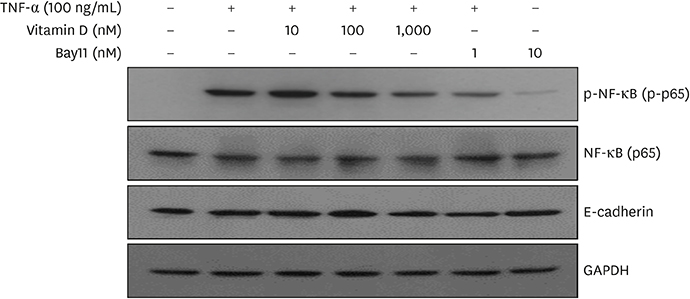
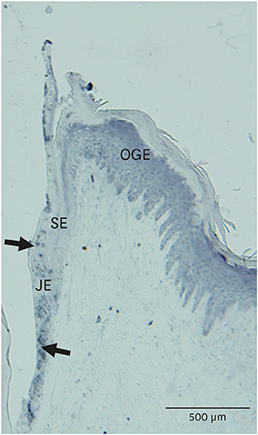
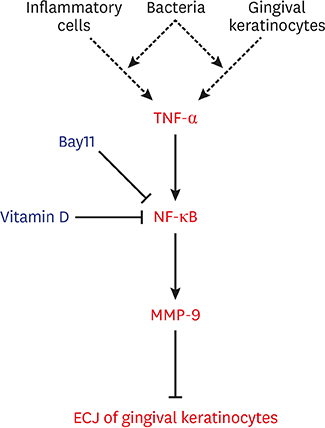
TNF-α downregulated the development of the ECJs of the HGKs. Dissociation of the ECJs by TNF-α was accompanied by the upregulation of MMP-9 production and suppressed by a specific MMP-9 inhibitor, Bay 11-7082. Exogenous MMP-9 decreased the development of ECJs. Vitamin D reduced the production of MMP-9 and attenuated the breakdown of ECJs in the HGKs treated with TNF-α. In addition, vitamin D downregulated TNF-α-induced nuclear factor kappa B (NF-κB) signaling in the HGKs. VDR was expressed in the gingival epithelium, including the junctional epithelium.
CONCLUSIONS
These results suggest that vitamin D may avert TNF-α-induced downregulation of the development of ECJs in HGKs by decreasing the production of MMP-9, which was upregulated by TNF-α. Vitamin D may reinforce ECJs by downregulating NF-κB signaling, which is upregulated by TNF-α. Strengthening the epithelial barrier may be a way for vitamin D to protect the periodontium from bacterial invasion.
Keyword
MeSH Terms
-
Bays
Blotting, Western
Cadherins*
Cell Line
Down-Regulation
Epithelial Attachment
Epithelium
Gelatin
Humans*
Intercellular Junctions*
Keratinocytes*
Matrix Metalloproteinase 9
Matrix Metalloproteinases
NF-kappa B
Periodontium
Receptors, Calcitriol
Tumor Necrosis Factor-alpha
Up-Regulation
Vitamin D*
Vitamins*
Cadherins
Gelatin
Matrix Metalloproteinase 9
Matrix Metalloproteinases
NF-kappa B
Receptors, Calcitriol
Tumor Necrosis Factor-alpha
Vitamin D
Vitamins
Figure
Reference
-
1. Alshouibi EN, Kaye EK, Cabral HJ, Leone CW, Garcia RI. Vitamin D and periodontal health in older men. J Dent Res. 2013; 92:689–693.
Article2. Lin Z, Li W. The roles of vitamin D and its analogs in inflammatory diseases. Curr Top Med Chem. 2016; 16:1242–1261.
Article3. Garcia MN, Hildebolt CF, Miley DD, Dixon DA, Couture RA, Spearie CL, et al. One-year effects of vitamin D and calcium supplementation on chronic periodontitis. J Periodontol. 2011; 82:25–32.
Article4. Dietrich T, Nunn M, Dawson-Hughes B, Bischoff-Ferrari HA. Association between serum concentrations of 25-hydroxyvitamin D and gingival inflammation. Am J Clin Nutr. 2005; 82:575–580.
Article5. Dietrich T, Joshipura KJ, Dawson-Hughes B, Bischoff-Ferrari HA. Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am J Clin Nutr. 2004; 80:108–113.6. Hiremath VP, Rao CB, Naik V, Prasad KV. Anti-inflammatory effect of vitamin D on gingivitis: a dose-response randomised control trial. Oral Health Prev Dent. 2013; 11:61–69.7. De Filippis A, Fiorentino M, Guida L, Annunziata M, Nastri L, Rizzo A. Vitamin D reduces the inflammatory response by Porphyromonas gingivalis infection by modulating human β-defensin-3 in human gingival epithelium and periodontal ligament cells. Int Immunopharmacol. 2017; 47:106–117.
Article8. McMahon L, Schwartz K, Yilmaz O, Brown E, Ryan LK, Diamond G. Vitamin D-mediated induction of innate immunity in gingival epithelial cells. Infect Immun. 2011; 79:2250–2256.
Article9. Bosshardt DD, Lang NP. The junctional epithelium: from health to disease. J Dent Res. 2005; 84:9–20.
Article10. Damek-Poprawa M, Korostoff J, Gill R, DiRienzo JM. Cell junction remodeling in gingival tissue exposed to a microbial toxin. J Dent Res. 2013; 92:518–523.
Article11. Hatakeyama S, Yaegashi T, Oikawa Y, Fujiwara H, Mikami T, Takeda Y, et al. Expression pattern of adhesion molecules in junctional epithelium differs from that in other gingival epithelia. J Periodontal Res. 2006; 41:322–328.
Article12. Bosshardt DD. The periodontal pocket: pathogenesis, histopathology and consequences. Periodontol 2000. 2018; 76:43–50.
Article13. Lee G, Kim HJ, Kim HM. RhoA-JNK regulates the E-cadherin junctions of human gingival epithelial cells. J Dent Res. 2016; 95:284–291.
Article14. Lewis JE, Wahl JK 3rd, Sass KM, Jensen PJ, Johnson KR, Wheelock MJ. Cross-talk between adherens junctions and desmosomes depends on plakoglobin. J Cell Biol. 1997; 136:919–934.
Article15. Ye P, Chapple CC, Kumar RK, Hunter N. Expression patterns of E-cadherin, involucrin, and connexin gap junction proteins in the lining epithelia of inflamed gingiva. J Pathol. 2000; 192:58–66.
Article16. Miyagawa T, Fujita T, Yumoto H, Yoshimoto T, Kajiya M, Ouhara K, et al. Azithromycin recovers reductions in barrier function in human gingival epithelial cells stimulated with tumor necrosis factor-α. Arch Oral Biol. 2016; 62:64–69.
Article17. Choi YS, Baek K, Choi Y. Estrogen reinforces barrier formation and protects against tumor necrosis factor alpha-induced barrier dysfunction in oral epithelial cells. J Periodontal Implant Sci. 2018; 48:284–294.
Article18. Park NH, Min BM, Li SL, Huang MZ, Cherick HM, Doniger J. Immortalization of normal human oral keratinocytes with type 16 human papillomavirus. Carcinogenesis. 1991; 12:1627–1631.
Article19. Jin C, Lee G, Oh C, Kim HJ, Kim HM. Substrate roughness induces the development of defective E-cadherin junctions in human gingival keratinocytes. J Periodontal Implant Sci. 2017; 47:116–131.
Article20. Symowicz J, Adley BP, Gleason KJ, Johnson JJ, Ghosh S, Fishman DA, et al. Engagement of collagen-binding integrins promotes matrix metalloproteinase-9-dependent E-cadherin ectodomain shedding in ovarian carcinoma cells. Cancer Res. 2007; 67:2030–2039.
Article21. Zheng G, Lyons JG, Tan TK, Wang Y, Hsu TT, Min D, et al. Disruption of E-cadherin by matrix metalloproteinase directly mediates epithelial-mesenchymal transition downstream of transforming growth factor-beta1 in renal tubular epithelial cells. Am J Pathol. 2009; 175:580–591.
Article22. Koli K, Keski-Oja J. 1α,25-dihydroxyvitamin D3 and its analogues down-regulate cell invasion-associated proteases in cultured malignant cells. Cell Growth Differ. 2000; 11:221–229.23. Bao BY, Yeh SD, Lee YF. 1α,25-dihydroxyvitamin D3 inhibits prostate cancer cell invasion via modulation of selective proteases. Carcinogenesis. 2006; 27:32–42.
Article24. Chiang KC, Yeh CN, Hsu JT, Jan YY, Chen LW, Kuo SF, et al. The vitamin D analog, MART-10, represses metastasis potential via downregulation of epithelial-mesenchymal transition in pancreatic cancer cells. Cancer Lett. 2014; 354:235–244.
Article25. Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017; 2:17023.
Article26. Yuan FN, Valiyaparambil J, Woods MC, Tran H, Pant R, Adams JS, et al. Vitamin D signaling regulates oral keratinocyte proliferation in vitro and in vivo. Int J Oncol. 2014; 44:1625–1633.
Article27. Fischer KD, Agrawal DK. Vitamin D regulating TGF-β induced epithelial-mesenchymal transition. Respir Res. 2014; 15:146.
Article28. Lopes N, Carvalho J, Durães C, Sousa B, Gomes M, Costa JL, et al. 1α,25-dihydroxyvitamin D3 induces de novo E-cadherin expression in triple-negative breast cancer cells by CDH1-promoter demethylation. Anticancer Res. 2012; 32:249–257.29. Larriba MJ, García de Herreros A, Muñoz A. Vitamin D and the epithelial to mesenchymal transition. Stem Cells Int. 2016; 2016:6213872.
Article30. Fujita T, Yumoto H, Shiba H, Ouhara K, Miyagawa T, Nagahara T, et al. Irsogladine maleate regulates epithelial barrier function in tumor necrosis factor-α-stimulated human gingival epithelial cells. J Periodontal Res. 2012; 47:55–61.
Article31. Halldorsson S, Gudjonsson T, Gottfredsson M, Singh PK, Gudmundsson GH, Baldursson O. Azithromycin maintains airway epithelial integrity during Pseudomonas aeruginosa infection. Am J Respir Cell Mol Biol. 2010; 42:62–68.
Article32. Carayol N, Campbell A, Vachier I, Mainprice B, Bousquet J, Godard P, et al. Modulation of cadherin and catenins expression by tumor necrosis factor-α and dexamethasone in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2002; 26:341–347.
Article33. Yi JY, Jung YJ, Choi SS, Chung E. TNF-alpha downregulates E-cadherin and sensitizes response to γ-irradiation in Caco-2 cells. Cancer Res Treat. 2009; 41:164–170.
Article34. Saito T, Yoshida K, Matsumoto K, Saeki K, Tanaka Y, Ong SM, et al. Inflammatory cytokines induce a reduction in E-cadherin expression and morphological changes in MDCK cells. Res Vet Sci. 2014; 96:288–291.
Article35. Vandooren J, Van den Steen PE, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): the next decade. Crit Rev Biochem Mol Biol. 2013; 48:222–272.
Article36. Ji S, Choi Y. Point-of-care diagnosis of periodontitis using saliva: technically feasible but still a challenge. Front Cell Infect Microbiol. 2015; 5:65.
Article37. Kim HD, Shin MS, Kim HT, Kim MS, Ahn YB. Incipient periodontitis and salivary molecules among Korean adults: association and screening ability. J Clin Periodontol. 2016; 43:1032–1040.
Article38. Hayden MS, Ghosh S. Regulation of NF-κB by TNF family cytokines. Semin Immunol. 2014; 26:253–266.
Article39. Zhao B, Li R, Yang F, Yu F, Xu N, Zhang F, et al. LPS-induced vitamin D receptor decrease in oral keratinocytes is associated with oral lichen planus. Sci Rep. 2018; 8:763.
Article40. Janjetovic Z, Zmijewski MA, Tuckey RC, DeLeon DA, Nguyen MN, Pfeffer LM, et al. 20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-κB activity by increasing IκBα levels in human keratinocytes. PLoS One. 2009; 4:e5988.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Substrate roughness induces the development of defective E-cadherin junctions in human gingival keratinocytes
- Effects of Interleukin 4 on the Production of Interleukin 6 in Human Keratinocytes
- The mRNA expression of MMP-1, TIMP-1, TGF-beta1 in gingival keratocytes from gingival hyperplasia induced by cyclosporine A
- The Expression of MT1-MMP and E-cadherin mRNA in Invasive Cervical Cancer
- The Effects of Calcium and Retinoic Acid on Epidermal Desmosomes