J Periodontal Implant Sci.
2017 Apr;47(2):116-131. 10.5051/jpis.2017.47.2.116.
Substrate roughness induces the development of defective E-cadherin junctions in human gingival keratinocytes
- Affiliations
-
- 1Laboratory for the Study of Molecular Biointerfaces, Department of Oral Histology and Developmental Biology, Program of Cell and Developmental Biology, Dental Research Institute, Seoul National University School of Dentistry, Seoul, Korea. hyunmkim@snu.ac
- KMID: 2377746
- DOI: http://doi.org/10.5051/jpis.2017.47.2.116
Abstract
- PURPOSE
The entry of bacteria or harmful substances through the epithelial seal of human gingival keratinocytes (HGKs) in the junctional epithelium (JE) is blocked by specialized intercellular junctions such as E-cadherin junctions (ECJs). However, the influence of roughened substrates, which may occur due to apical migration of the JE, root planing, or peri-implantitis, on the development of the ECJs of HGKs remains largely unknown.
METHODS
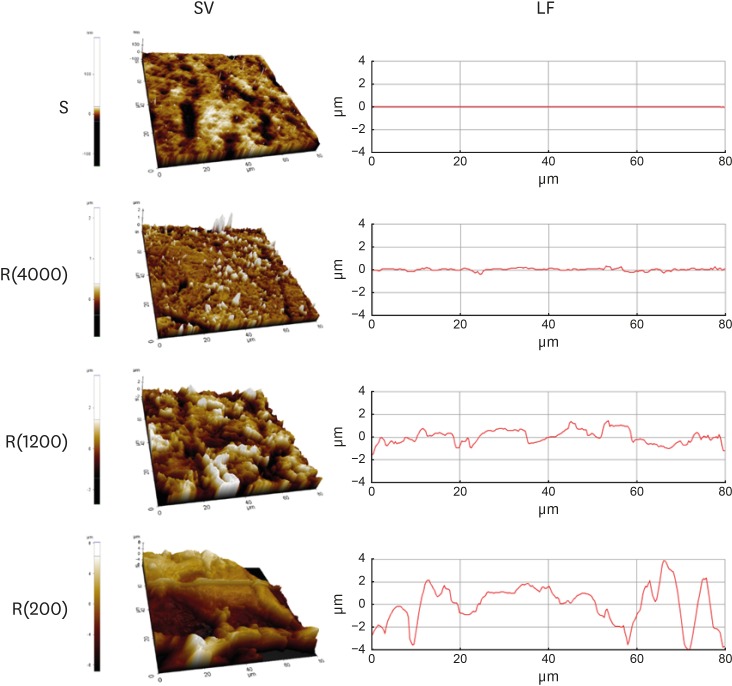
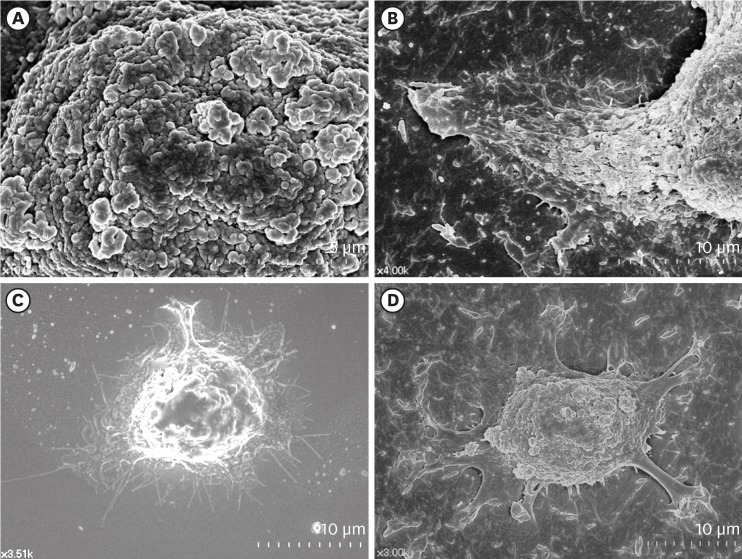
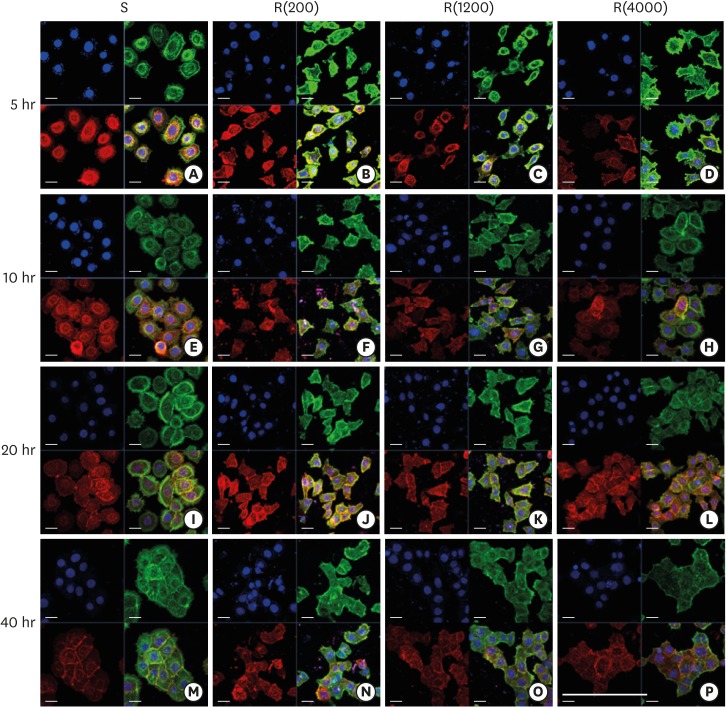
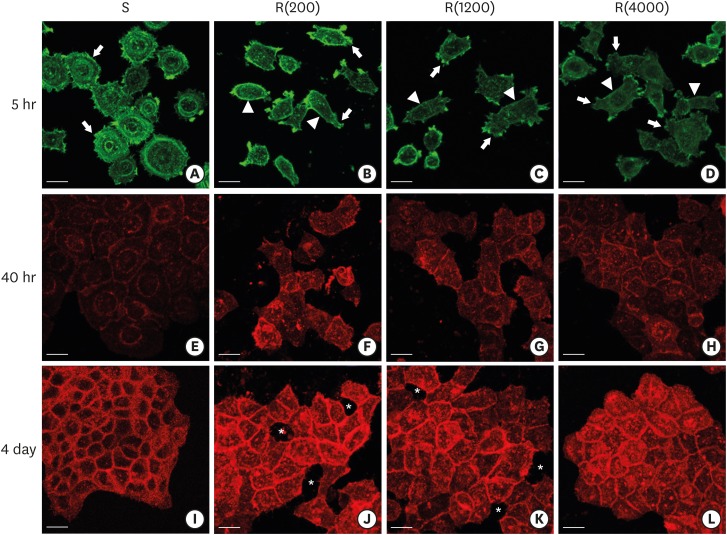
HGKs were cultured on substrates with varying levels of roughness, which were prepared by rubbing hydrophobic polystyrene dishes with silicon carbide papers. The activity of c-Jun N-terminal kinase (JNK) was inhibited with SP600125 or by transfection with JNK short hairpin RNA. The development of intercellular junctions was analyzed using scanning electron microscopy or confocal laser scanning microscopy after immunohistochemical staining of the cells for E-cadherin. The expression level of phospho-JNK was assessed by immunoblotting.
RESULTS
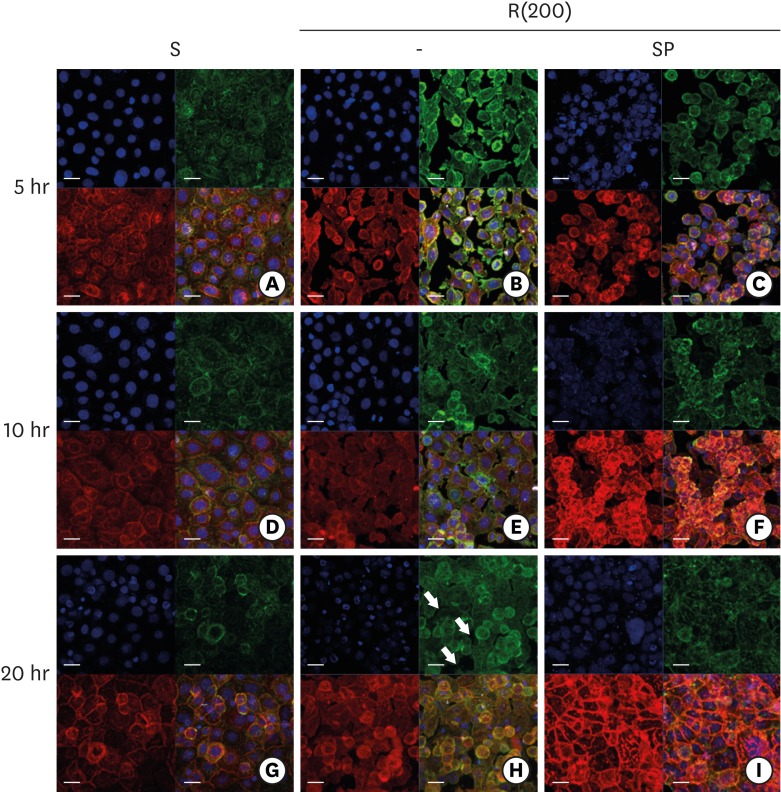
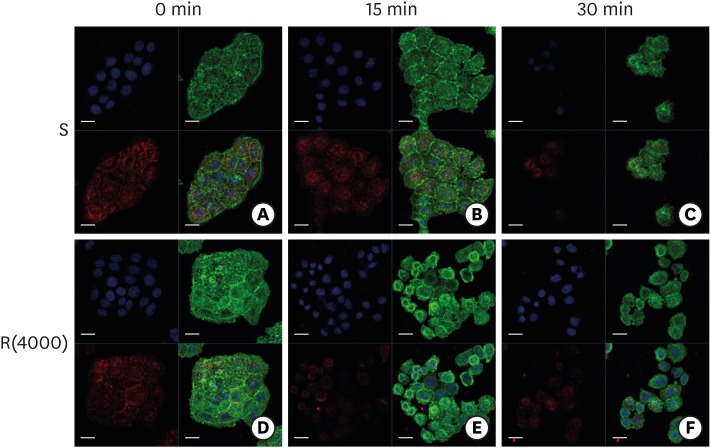
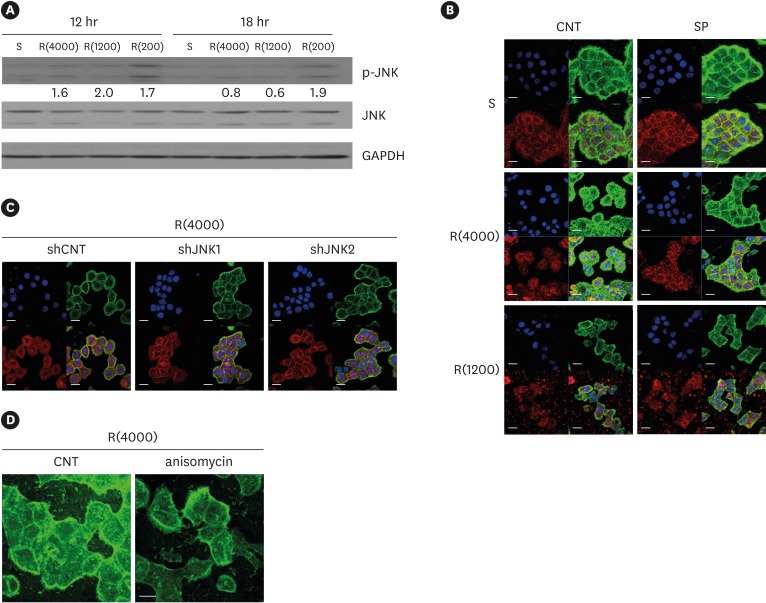
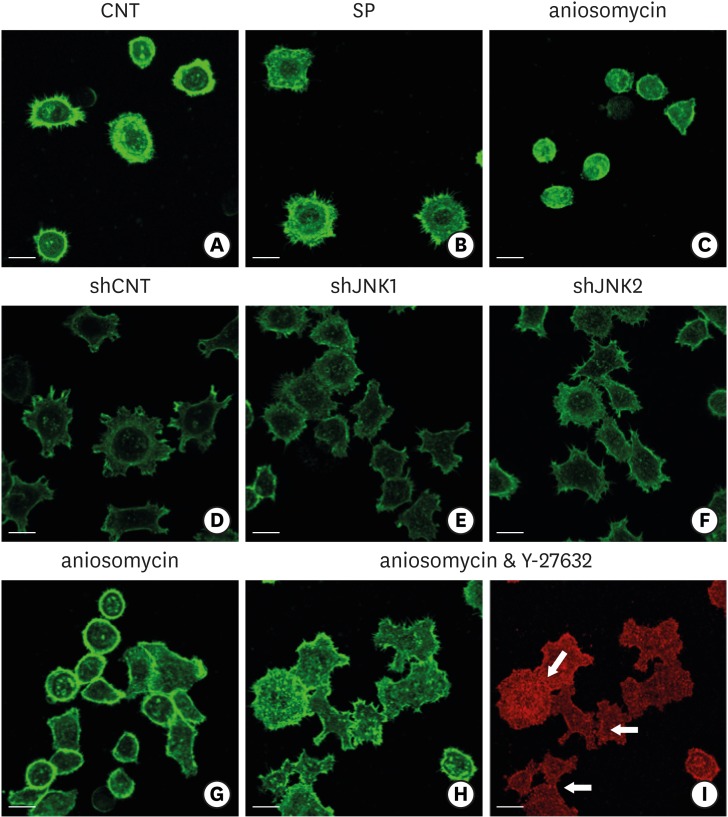
HGKs developed tight intercellular junctions devoid of wide intercellular gaps on smooth substrates and on rough substrates with low-nanometer dimensions (average roughness [Ra]=121.3±13.4 nm), although the ECJs of HGKs on rough substrates with low-nanometer dimensions developed later than those of HGKs on smooth substrates. In contrast, HGKs developed short intercellular junctions with wide intercellular gaps on rough substrates with mid- or high-nanometer dimensions (Ra=505.3±115.3 nm, 867.0±168.6 nm). Notably, the stability of the ECJs was low on the rough substrates, as demonstrated by the rapid destruction of the cell junction following calcium depletion. Inhibition of JNK activity promoted ECJ development in HGKs. JNK was closely associated with cortical actin in the regulation of ECJs in HGKs.
CONCLUSIONS
These results indicate that on rough substrates with nanometer dimensions, the ECJs of HGKs develop slowly or defectively, and that this effect can be reversed by inhibiting JNK.
Keyword
MeSH Terms
-
Actins
Bacteria
Cadherins*
Calcium
Dental Implants
Epithelial Attachment
Humans*
Immunoblotting
Intercellular Junctions
JNK Mitogen-Activated Protein Kinases
Keratinocytes*
Microscopy, Confocal
Microscopy, Electron, Scanning
Peri-Implantitis
Periodontal Diseases
Polystyrenes
Re-Epithelialization
RNA, Small Interfering
Root Planing
Silicon
Transfection
Actins
Cadherins
Calcium
Dental Implants
JNK Mitogen-Activated Protein Kinases
Polystyrenes
RNA, Small Interfering
Silicon
Figure
Reference
-
1. Bosshardt DD, Lang NP. The junctional epithelium: from health to disease. J Dent Res. 2005; 84:9–20. PMID: 15615869.
Article2. Hara AT, Livengood SV, Lippert F, Eckert GJ, Ungar PS. Dental surface texture characterization based on erosive tooth wear processes. J Dent Res. 2016; 95:537–542. PMID: 26848070.
Article3. Merijohn GK. Management and prevention of gingival recession. Periodontol 2000. 2016; 71:228–242. PMID: 27045439.
Article4. Solís Moreno C, Santos A, Nart J, Levi P, Velásquez A, Sanz Moliner J. Evaluation of root surface microtopography following the use of four instrumentation systems by confocal microscopy and scanning electron microscopy: an in vitro study. J Periodontal Res. 2012; 47:608–615. PMID: 22494068.5. Amid R, Kadkhodazadeh M, Fekrazad R, Hajizadeh F, Ghafoori A. Comparison of the effect of hand instruments, an ultrasonic scaler, and an erbium-doped yttrium aluminium garnet laser on root surface roughness of teeth with periodontitis: a profilometer study. J Periodontal Implant Sci. 2013; 43:101–105. PMID: 23678394.
Article6. Santos FA, Pochapski MT, Leal PC, Gimenes-Sakima PP, Marcantonio E Jr. Comparative study on the effect of ultrasonic instruments on the root surface in vivo . Clin Oral Investig. 2008; 12:143–150.7. Lee HJ, Lee J, Lee JT, Hong JS, Lim BS, Park HJ, et al. Microgrooves on titanium surface affect peri-implant cell adhesion and soft tissue sealing; an in vitro and in vivo study. J Periodontal Implant Sci. 2015; 45:120–126. PMID: 26131372.8. Lee HJ, Yang IH, Kim SK, Yeo IS, Kwon TK. In vivo comparison between the effects of chemically modified hydrophilic and anodically oxidized titanium surfaces on initial bone healing. J Periodontal Implant Sci. 2015; 45:94–100. PMID: 26131369.9. Lee G, Kim HJ, Kim HM. RhoA-JNK regulates the E-cadherin junctions of human gingival epithelial cells. J Dent Res. 2016; 95:284–291. PMID: 26635280.
Article10. Zheng L, Kim HM. Low-Rac1 activity downregulates MC3T3-E1 osteoblastic cell motility on a nanoscale topography prepared on polystyrene substrates in vitro . J Biomed Mater Res A. 2013; 101:1629–1636. PMID: 23184573.11. Torres-Gallegos I, Zavala-Alonso V, Patiño-Marín N, Martinez-Castañon GA, Anusavice K, Loyola-Rodríguez JP. Enamel roughness and depth profile after phosphoric acid etching of healthy and fluorotic enamel. Aust Dent J. 2012; 57:151–156. PMID: 22624754.
Article12. Barkmeier WW, Erickson RL, Kimmes NS, Latta MA, Wilwerding TM. Effect of enamel etching time on roughness and bond strength. Oper Dent. 2009; 34:217–222. PMID: 19363978.
Article13. Singh S, Uppoor A, Nayak D. A comparative evaluation of the efficacy of manual, magnetostrictive and piezoelectric ultrasonic instruments--an in vitro profilometric and SEM study. J Appl Oral Sci. 2012; 20:21–26. PMID: 22437673.14. Vastardis S, Yukna RA, Rice DA, Mercante D. Root surface removal and resultant surface texture with diamond-coated ultrasonic inserts: an in vitro and SEM study. J Clin Periodontol. 2005; 32:467–473. PMID: 15842261.15. Park NH, Min BM, Li SL, Huang MZ, Cherick HM, Doniger J. Immortalization of normal human oral keratinocytes with type 16 human papillomavirus. Carcinogenesis. 1991; 12:1627–1631. PMID: 1654226.
Article16. Samarin SN, Ivanov AI, Flatau G, Parkos CA, Nusrat A. Rho/Rho-associated kinase-II signaling mediates disassembly of epithelial apical junctions. Mol Biol Cell. 2007; 18:3429–3439. PMID: 17596509.
Article17. Lecuit T. “Developmental mechanics”: cellular patterns controlled by adhesion, cortical tension and cell division. HFSP J. 2008; 2:72–78. PMID: 19404474.
Article18. Maghzal N, Kayali HA, Rohani N, Kajava AV, Fagotto F. EpCAM controls actomyosin contractility and cell adhesion by direct inhibition of PKC. Dev Cell. 2013; 27:263–277. PMID: 24183651.
Article19. Sahai E, Marshall CJ. ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nat Cell Biol. 2002; 4:408–415. PMID: 11992112.
Article20. Huang C, Rajfur Z, Borchers C, Schaller MD, Jacobson K. JNK phosphorylates paxillin and regulates cell migration. Nature. 2003; 424:219–223. PMID: 12853963.
Article21. Otto IM, Raabe T, Rennefahrt UE, Bork P, Rapp UR, Kerkhoff E. The p150-Spir protein provides a link between c-Jun N-terminal kinase function and actin reorganization. Curr Biol. 2000; 10:345–348. PMID: 10744979.
Article22. Samak G, Gangwar R, Crosby LM, Desai LP, Wilhelm K, Waters CM, et al. Cyclic stretch disrupts apical junctional complexes in Caco-2 cell monolayers by a JNK-2-, c-Src-, and MLCK-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2014; 306:G947–G958. PMID: 24722904.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vitamin D maintains E-cadherin intercellular junctions by downregulating MMP-9 production in human gingival keratinocytes treated by TNF-α
- Effects of substrates on the indirect immunofluorescence test for pemphigus vulgaris autoantibodies
- Bacteroides fragilis Toxin Induces IL-8 Secretion in HT29/C1 Cells through Disruption of E-cadherin Junctions
- Reduced Expression of E-cadherin in Human Non-small Cell Lung Carcinoma
- Expression of E-cadherin and alpha - , beta - , gamma - catenin proteins in endometrial carcinoma