Ann Lab Med.
2020 Mar;40(2):122-130. 10.3343/alm.2020.40.2.122.
Performance Evaluation of Body Fluid Cellular Analysis Using the Beckman Coulter UniCel DxH 800, Sysmex XN-350, and UF-5000 Automated Cellular Analyzers
- Affiliations
-
- 1Department of Laboratory Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. jeongho@yuhs.ac
- 2Department of Laboratory Medicine, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 3Department of Laboratory Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2460909
- DOI: http://doi.org/10.3343/alm.2020.40.2.122
Abstract
- BACKGROUND
Automated cellular analyzers are expected to improve the analytical performance in body fluid (BF) analysis. We evaluated the analytical performance of three automated cellular analyzers and established optimum reflex analysis guidelines.
METHODS
A total of 542 BF samples (88 cerebrospinal fluid [CSF] samples and 454 non-CSF samples) were examined using manual counting and three automated cellular analyzers: UniCel DxH 800 (Beckman Coulter), XN-350 (Sysmex), and UF-5000 (Sysmex). Additionally, 2,779 BF analysis results were retrospectively reviewed. For malignant cell analysis, the receiver operating characteristic (ROC) curve was used, and the detection of high fluorescence-BF cells (HF-BFs) using the XN-350 analyzer was compared with cytology results.
RESULTS
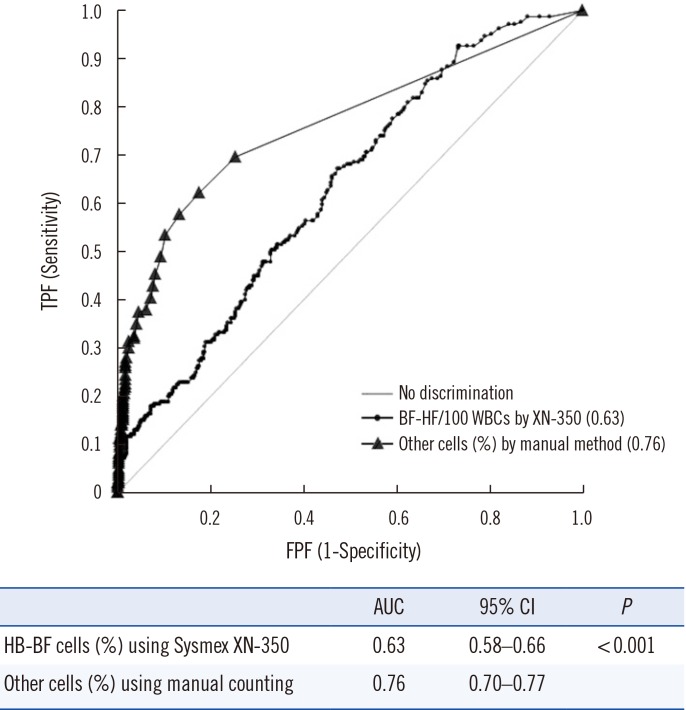
All three analyzers showed good agreement for total nucleated cell (TNC) and red blood cell (RBC) counts, except for the RBC count in CSF samples using the UniCel DxH 800. However, variable degrees of differences were observed during differential cell counting. For malignant cell analysis, the area under the curve was 0.63 for the XN-350 analyzer and 0.76 for manual counting. We established our own reflex analysis guidelines as follows: HF-BFs <0.7/100 white blood cells (WBCs) is the criterion for quick scans with 100× magnification microscopic examination as a rule-out cut-off, while HF-BFs >83.4/100 WBCs or eosinophils >3.8% are the criteria for mandatory double check confirmation with 1,000× magnification examination.
CONCLUSIONS
The three automated analyzers showed good analytical performances. Application of reflex analysis guidelines is recommended for eosinophils and HF-BFs, and manual confirmation is warranted.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Obtaining Reliable CBC Results in Clinical Laboratories
Seon Young Kim, Hyun Kyung Kim
Ann Lab Med. 2022;42(5):505-506. doi: 10.3343/alm.2022.42.5.505.Body Fluid Analysis for Cellular Composition Using Manual Methods: Current Status and Clinical Laboratory Guidelines in Korea (2021)
Hae In Bang, Hyun-Young Kim, Saeam Shin, Ja Young Lee, In-Suk Kim, Young-Uk Cho, Ji Myung Kim, Myung-Geun Shin, Jeong Nyeo Lee, Sang Mee Hwang, Sun-Young Kong
Lab Med Online. 2022;12(4):262-268. doi: 10.47429/lmo.2022.12.4.262.
Reference
-
1. Sandhaus LM. Body fluid cell counts by automated methods. Clin Lab Med. 2015; 35:93–103. PMID: 25676374.2. Fleming C, Russcher H, Lindemans J, de Jonge R. Clinical relevance and contemporary methods for counting blood cells in body fluids suspected of inflammatory disease. Clin Chem Lab Med. 2015; 53:1689–1706. PMID: 25879321.3. Yang D, Zhou Y, Chen B. Performance evaluation and result comparison of the automated hematology analyzers Abbott CD 3700, Sysmex XE 2100 and Coulter LH 750 for cell counts in serous fluids. Clin Chim Acta. 2013; 419:113–118. PMID: 23415694.4. CLSI. Body fluid analysis for cellular composition; Approved guideline. CLSI H56-A. Wayne, PA: Clinical and Laboratory Standards Institute;2006.5. Cho YU, You E, Jang S, Park CJ. Validation of reflex testing rules and establishment of a new workflow for body fluid cell analysis using a Sysmex XN-550 automatic hematology analyzer. Int J Lab Hematol. 2018; 40:258–267. PMID: 29314650.6. Fleming C, Brouwer R, van Alphen A, Lindemans J, de Jonge R. UF-1000i: validation of the body fluid mode for counting cells in body fluids. Clin Chem Lab Med. 2014; 52:1781–1790. PMID: 24964259.7. Nanos NE, Delanghe JR. Evaluation of Sysmex UF-1000i for use in cerebrospinal fluid analysis. Clin Chim Acta. 2008; 392:30–33. PMID: 18348868.8. Cho YU, Chi HS, Park SH, Jang S, Kim YJ, Park CJ. Body fluid cellular analysis using the Sysmex XN-2000 automatic hematology analyzer: focusing on malignant samples. Int J Lab Hematol. 2015; 37:346–356. PMID: 25212101.9. Bourner G, De la Salle B, George T, Tabe Y, Baum H, Culp N, et al. ICSH guidelines for the verification and performance of automated cell counters for body fluids. Int J Lab Hematol. 2014; 36:598–612. PMID: 24628711.10. Sandhaus LM, Dillman CA, Hinkle WP, MacKenzie JM, Hong G. A new automated technology for cerebrospinal fluid cell counts: comparison of accuracy and clinical impact of GloCyte, Sysmex XN, and manual methods. Am J Clin Pathol. 2017; 147:507–514. PMID: 28419185.11. Fleming C, Russcher H, Brouwer R, Lindemans J, de Jonge R. Evaluation of Sysmex XN-1000 high-sensitive analysis (hsA) research mode for counting and differentiating cells in cerebrospinal fluid. Am J Clin Pathol. 2016; 145:299–307. PMID: 27124911.12. Danise P, Maconi M, Rovetti A, Avino D, Di Palma A, Gerardo Pirofalo M, et al. Cell counting of body fluids: comparison between three automated haematology analysers and the manual microscope method. Int J Lab Hematol. 2013; 35:608–613. PMID: 23647736.13. Lehto TM, Leskinen P, Hedberg P, Vaskivuo TE. Evaluation of the Sysmex XT-4000i for the automated body fluid analysis. Int J Lab Hematol. 2014; 36:114–123.14. Fleming C, Brouwer R, Lindemans J, de Jonge R. Validation of the body fluid module on the new Sysmex XN-1000 for counting blood cells in cerebrospinal fluid and other body fluids. Clin Chem Lab Med. 2012; 50:1791–1798. PMID: 23089709.15. Labaere D, Boeckx N, Geerts I, Moens M, Van den Driessche M. Detection of malignant cells in serous body fluids by counting high-fluorescent cells on the Sysmex XN-2000 hematology analyzer. Int J Lab Hematol. 2015; 37:715–722. PMID: 26074270.16. Beckman Coulter Inc. UniCel DxH 800. Coulter cellular analysis system instructions cellular analysis system instructions for use: use: PN B26647AE. Brea, CA: Beckman Coulter, Inc;2017. p. 127.17. Previtali G, Ravasio R, Seghezzi M, Buoro S, Alessio MG. Performance evaluation of the new fully automated urine particle analyser UF-5000 compared to the reference method of the Fuchs-Rosenthal chamber. Clin Chim Acta. 2017; 472:123–130. PMID: 28760666.18. Seghezzi M, Manenti B, Previtali G, Alessio MG, Dominoni P, Buoro S. Preliminary evaluation of UF-5000 Body Fluid Mode for automated cerebrospinal fluid cell counting. Clin Chim Acta. 2017; 473:133–138. PMID: 28843601.19. Buoro S, Apassiti Esposito S, Alessio M, Crippa A, Ottomano C, Lippi G. Automated cerebrospinal fluid cell counts using cerebrospinal fluid cell counts using the new body fluid mode of Sysmex UF-1000i. J Clin Lab Anal. 2016; 30:381–391. PMID: 26302990.20. CLSI. User verification of performance for precision and trueness approved guideline. 2nd ed. CLSI 15-A2. Wayne. PA: Clinical and Laboratory Standards Institute;2006.21. CLSI. Evaluation of the linearity of quantitative measurement procedures: a statistical approach; approved guideline. CLSI approach approved guideline. CLSI EP06-A. Wayne, PA: Clinical and Laboratory Standards Institute;2003.22. CLSI. Evaluation of detection capability for clinical laboratory measurement procedures; approved guideline. 2nd ed. CLSI measurement procedures approved guideline. CLSI 17-A2. Wayne. PA: Clinical and Laboratory Standards Institute;2012.23. Westgard QC. Desirable biological variation database specifications. Updated on Mar 2019. https://www.westgard.com/biodatabase1.htm.24. CLSI. Method comparison and bias estimation using patient samples; approved guideline. 2nd ed. (interim revision). CLSI 09-A2-IR. Wayne, PA: Clinical and Laboratory Standards Institute;2010.25. Buoro S, Appassiti Esposito S, Vavassori M, Mecca T, Ottomano C, Dominoni P, et al. Reflex testing rules for cell count and differentiation of nucleated elements in pleural and ascitic fluids on Sysmex XE-5000. J Lab Autom. 2016; 21:297–304. PMID: 26149816.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Performance Evaluation of the Beckman Coulter DxH 900 Automated Hematology Analyzer
- Comparison of Red Blood Cell, White Blood Cell and Differential Counts between UF-5000 System and Manual Method
- Differential Blast Counts Obtained by Automated Blood Cell Analyzers
- Performance Evaluation of the DxH 800 Hematology Analyzer
- Comparison of IRIS Iq200, UF-1000i, and Cobas u701 Module Automated Urine Sediment Analyzers