Cancer Res Treat.
2019 Oct;51(4):1275-1284. 10.4143/crt.2018.569.
Nomogram Development and External Validation for Predicting the Risk of Lymph Node Metastasis in T1 Colorectal Cancer
- Affiliations
-
- 1Center for Colorectal Cancer, Research Institute and Hospital, National Cancer Center, Goyang, Korea. kshan@ncc.re.kr
- 2Biometrics Research Branch, Research Institute and Hospital, National Cancer Center, Goyang, Korea.
- 3Department of Surgery, Daehang Hospital, Seoul, Korea.
- KMID: 2460577
- DOI: http://doi.org/10.4143/crt.2018.569
Abstract
- PURPOSE
Predicting lymph node metastasis (LNM) risk is crucial in determining further treatment strategies following endoscopic resection of T1 colorectal cancer (CRC). This study aimed to establish a new prediction model for the risk of LNM in T1 CRC patients.
MATERIALS AND METHODS
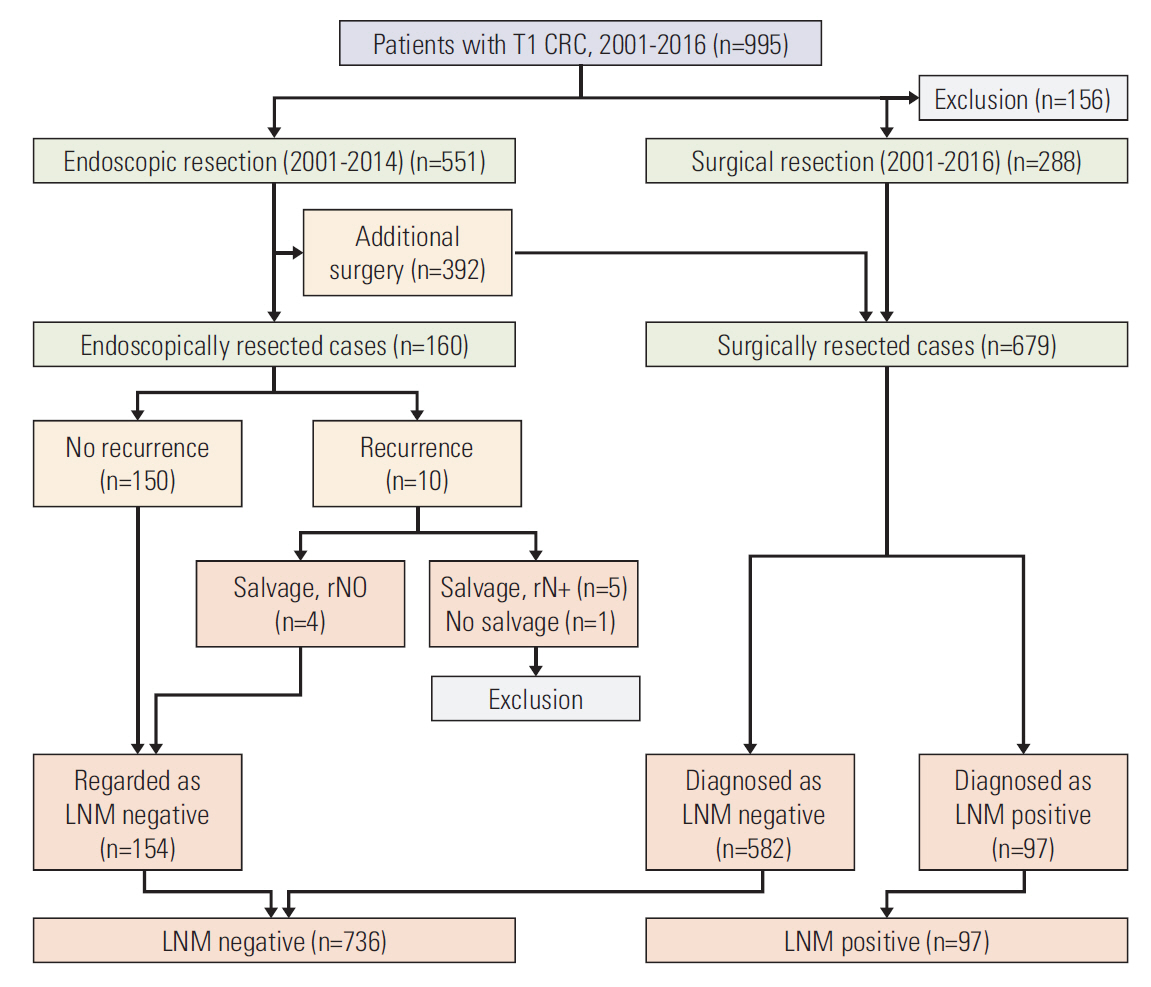
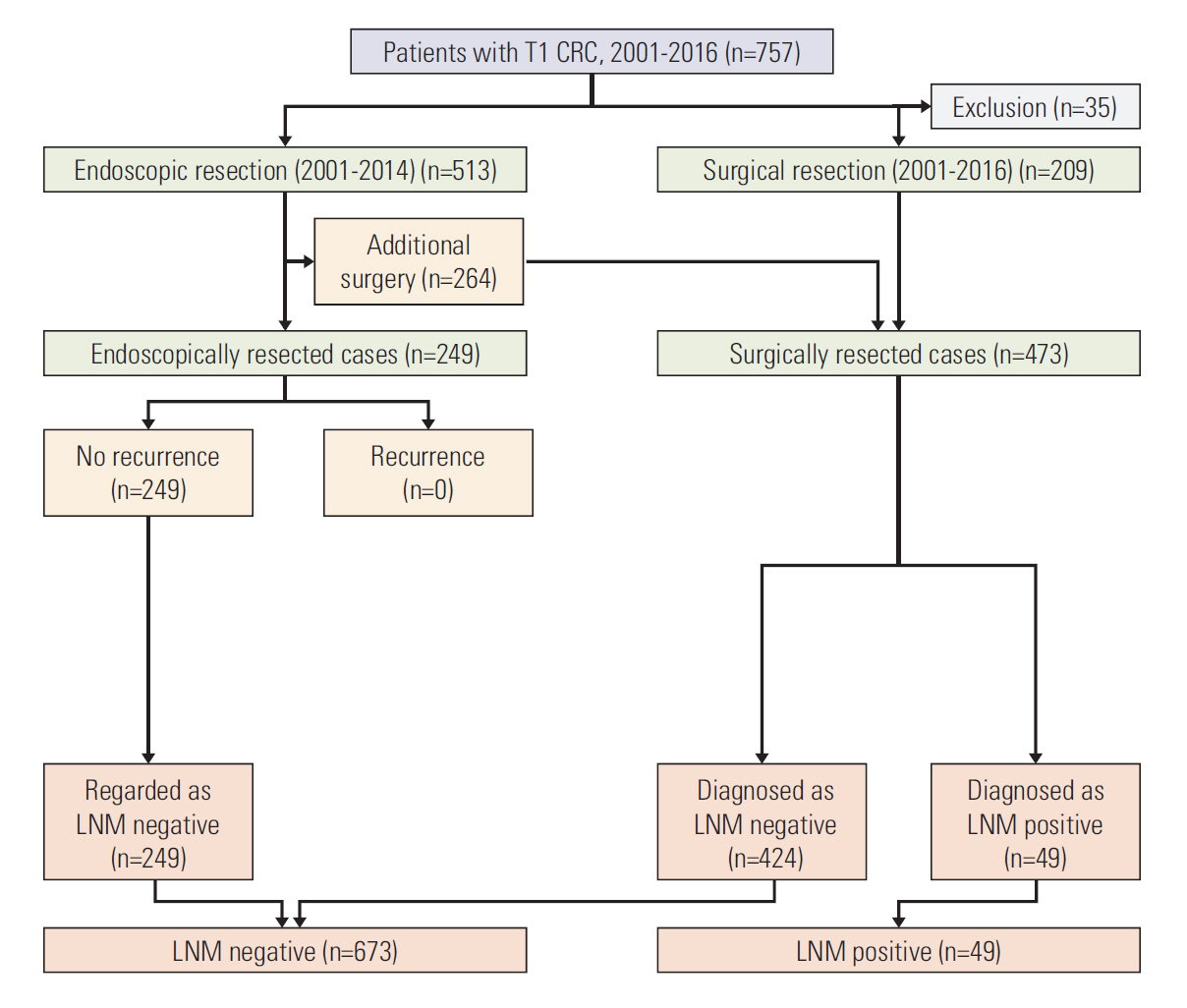
The development set included 833 patients with T1 CRC who had undergone endoscopic (n=154) or surgical (n=679) resection at the National Cancer Center. The validation set included 722 T1 CRC patients who had undergone endoscopic (n=249) or surgical (n=473) resection at Daehang Hospital. A logistic regression model was used to construct the prediction model. To assess the performance of prediction model, discrimination was evaluated using the receiver operating characteristic (ROC) curves with area under the ROC curve (AUC), and calibration was assessed using the Hosmer-Lemeshow (HL) goodness-of-fit test.
RESULTS
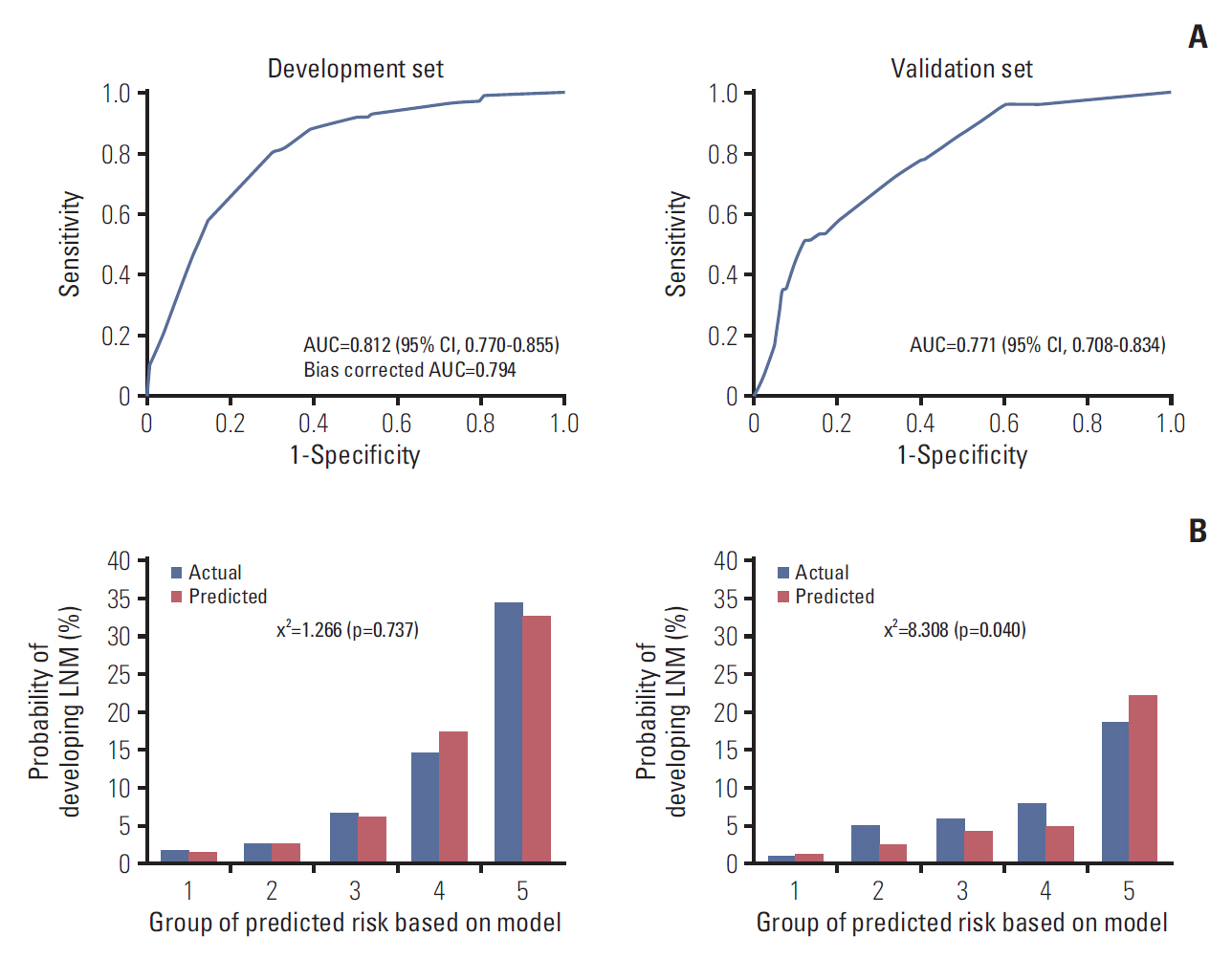
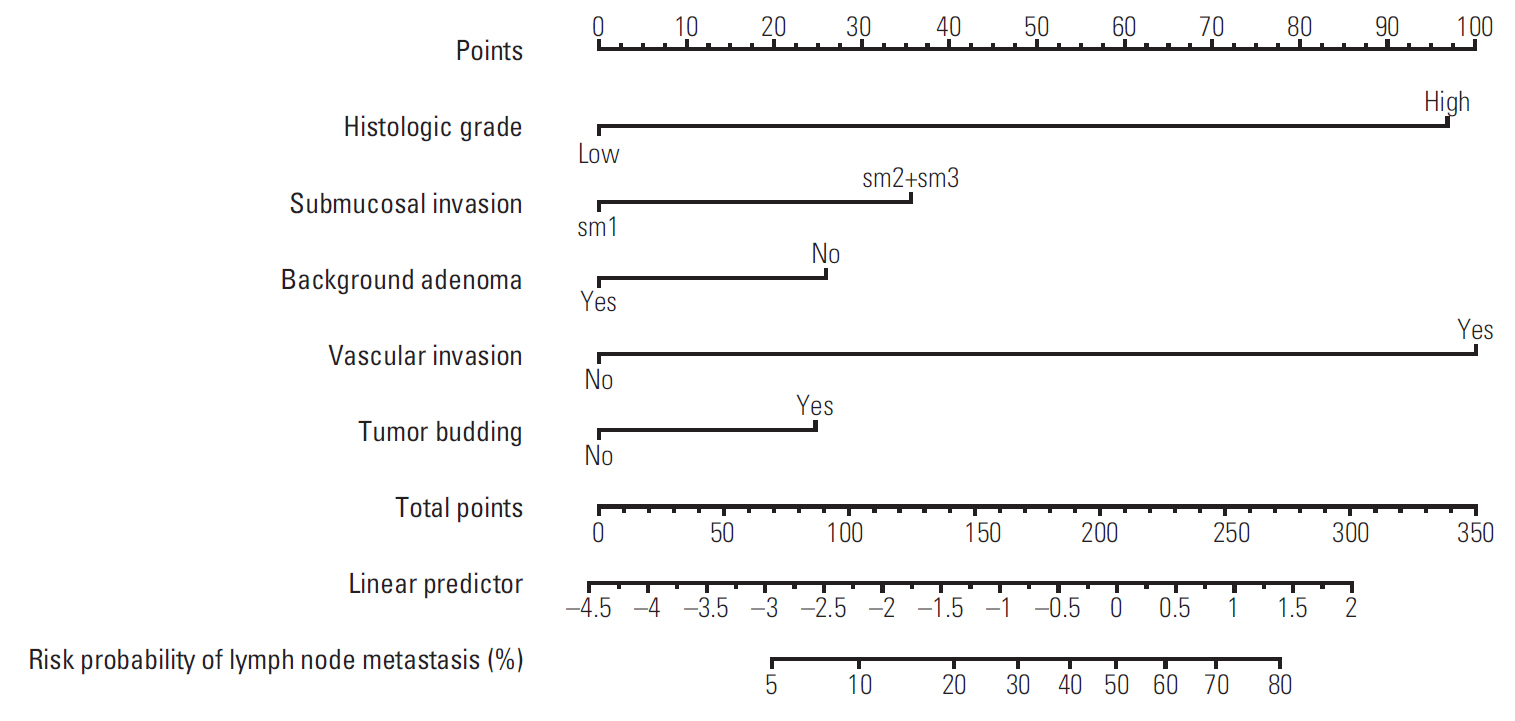
Five independent risk factors were determined in the multivariable model, including vascular invasion, high-grade histology, submucosal invasion, budding, and background adenoma. In final prediction model, the performance of the model was good that the AUC was 0.812 (95% confidence interval [CI], 0.770 to 0.855) and the HL chi-squared test statistic was 1.266 (p=0.737). In external validation, the performance was still good that the AUC was 0.771 (95% CI, 0.708 to 0.834) and the p-value of the HL chi-squared test was 0.040. We constructed the nomogram with the final prediction model.
CONCLUSION
We presented an externally validated new prediction model for LNM risk in T1 CRC patients, guiding decision making in determining whether additional surgery is required after endoscopic resection of T1 CRC.
MeSH Terms
Figure
Cited by 1 articles
-
LASSO-Based Machine Learning Algorithm for Prediction of Lymph Node Metastasis in T1 Colorectal Cancer
Jeonghyun Kang, Yoon Jung Choi, Im-kyung Kim, Hye Sun Lee, Hogeun Kim, Seung Hyuk Baik, Nam Kyu Kim, Kang Young Lee
Cancer Res Treat. 2021;53(3):773-783. doi: 10.4143/crt.2020.974.
Reference
-
References
1. Kikuchi R, Takano M, Takagi K, Fujimoto N, Nozaki R, Fujiyoshi T, et al. Management of early invasive colorectal cancer: risk of recurrence and clinical guidelines. Dis Colon Rectum. 1995; 38:1286–95.2. Nascimbeni R, Burgart LJ, Nivatvongs S, Larson DR. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum. 2002; 45:200–6.
Article3. Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, Kawamata H, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. 2004; 39:534–43.
Article4. Sohn DK, Chang HJ, Park JW, Choi DH, Han KS, Hong CW, et al. Histopathological risk factors for lymph node metastasis in submucosal invasive colorectal carcinoma of pedunculated or semipedunculated type. J Clin Pathol. 2007; 60:912–5.
Article5. Suh JH, Han KS, Kim BC, Hong CW, Sohn DK, Chang HJ, et al. Predictors for lymph node metastasis in T1 colorectal cancer. Endoscopy. 2012; 44:590–5.
Article6. Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol. 2015; 20:207–39.
Article7. Ueno H, Mochizuki H, Hashiguchi Y, Shimazaki H, Aida S, Hase K, et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004; 127:385–94.
Article8. Choi DH, Sohn DK, Chang HJ, Lim SB, Choi HS, Jeong SY. Indications for subsequent surgery after endoscopic resection of submucosally invasive colorectal carcinomas: a prospective cohort study. Dis Colon Rectum. 2009; 52:438–45.9. Tateishi Y, Nakanishi Y, Taniguchi H, Shimoda T, Umemura S. Pathological prognostic factors predicting lymph node metastasis in submucosal invasive (T1) colorectal carcinoma. Mod Pathol. 2010; 23:1068–72.
Article10. ASGE Standards of Practice Committee, Fisher DA, Shergill AK, Early DS, Acosta RD, Chandrasekhara V, et al. Role of endoscopy in the staging and management of colorectal cancer. Gastrointest Endosc. 2013; 78:8–12.
Article11. Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015; 47:829–54.
Article12. National Comprehensive Cancer Network clinical practice guidelines in oncology (NCCN guidelines): colon cancer. Version 2. 2016 [Internet]. Fort Washington, PA: National Comprehensive Cancer Network;c2017. [cited 2017 Jan 5]. Available from: http://www.nccn.org.professionals/physician_gls/f_guidelines.asp.13. National Comprehensive Cancer Network clinical practice guidelines in oncology (NCCN guidelines): rectal cancer. Version 1. 2016 [Internet]. Fort Washington, PA: National Comprehensive Cancer Network;c2017. [cited 2017 Jan 5]. Available from: http://www.nccn.org.professionals/physician_gls/f_guidelines.asp.14. Ha RK, Han KS, Sohn DK, Kim BC, Hong CW, Chang HJ, et al. Histopathologic risk factors for lymph node metastasis in patients with T1 colorectal cancer. Ann Surg Treat Res. 2017; 93:266–71.
Article15. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003; 58(6 Suppl):S3–43.16. Hamilton SR, Aaltonen LA. World Health Organization classification of tumors: pathology and genetics of tumors of the digestive system. Lyon: IARC Press;2000.17. Ueno H, Price AB, Wilkinson KH, Jass JR, Mochizuki H, Talbot IC. A new prognostic staging system for rectal cancer. Ann Surg. 2004; 240:832–9.
Article18. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996; 15:361–87.
Article19. Han KS, Lim SW, Sohn DK, Chang HJ, Oh JH, Lee JH, et al. Clinicopathological characteristics of T1 colorectal cancer without background adenoma. Colorectal Dis. 2013; 15:e124–9.
Article20. Ikematsu H, Yoda Y, Matsuda T, Yamaguchi Y, Hotta K, Kobayashi N, et al. Long-term outcomes after resection for submucosal invasive colorectal cancers. Gastroenterology. 2013; 144:551–9.
Article21. Nam MJ, Han KS, Kim BC, Hong CW, Sohn DK, Chang HJ, et al. Long-term outcomes of locally or radically resected T1 colorectal cancer. Colorectal Dis. 2016; 18:852–60.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Risk Stratification of T1 Colorectal Cancer Metastasis to Lymph Nodes: Current Status and Perspective
- Use of artificial intelligence in the management of T1 colorectal cancer: a new tool in the arsenal or is deep learning out of its depth?
- Accuracy Goals in Predicting Preoperative Lymph Node Metastasis for T1 Colorectal Cancer Resected Endoscopically
- Nomogram for Predicting Central Lymph Node Metastasis in Papillary Thyroid Cancer: A Retrospective Cohort Study of Two Clinical Centers
- Predictive value of chest computed tomography for axillary lymph node metastasis in patients with breast cancer A retrospective cohort study