J Pathol Transl Med.
2019 Sep;53(5):298-307. 10.4132/jptm.2019.07.15.
Reclassification of Mongolian Diffuse Gliomas According to the Revised 2016 World Health Organization Central Nervous System Tumor Classification
- Affiliations
-
- 1Department of Pathology, School of Biomedicine, Mongolian National University of Medical Sciences, Ulaanbaatar, Mongolia.
- 2Department of Pathology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea. gychoe@snubh.org
- KMID: 2459578
- DOI: http://doi.org/10.4132/jptm.2019.07.15
Abstract
- BACKGROUND
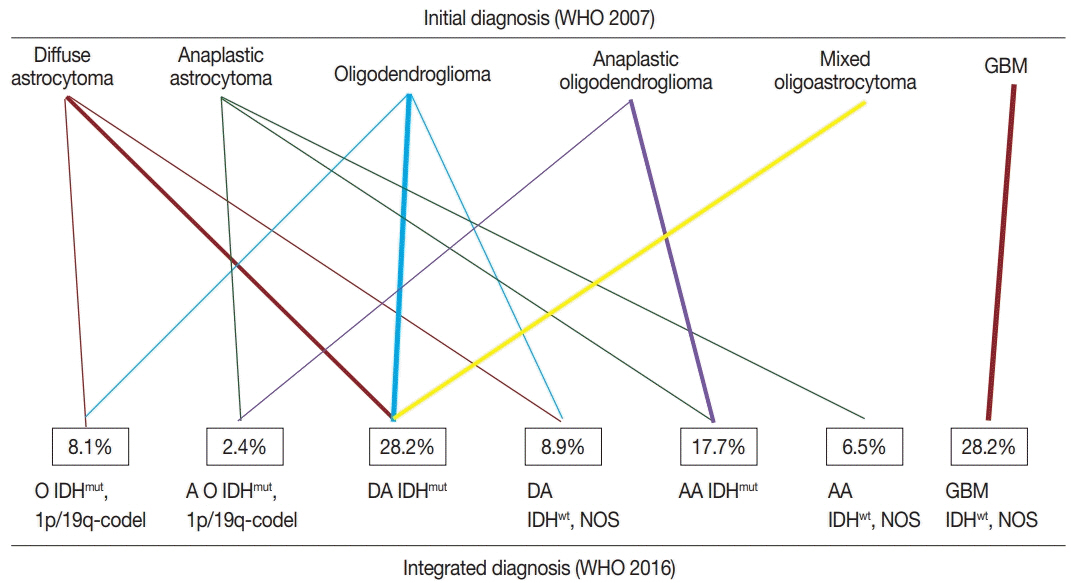
The 2016 World Health Organization (WHO) classification of central nervous system (CNS) tumors has been modified to incorporate the IDH mutation and 1p/19q co-deletion in the diagnosis of diffuse gliomas. In this study, we aimed to evaluate the feasibility and prognostic significance of the revised 2016 WHO classification of CNS tumors in Mongolian patients with diffuse gliomas.
METHODS
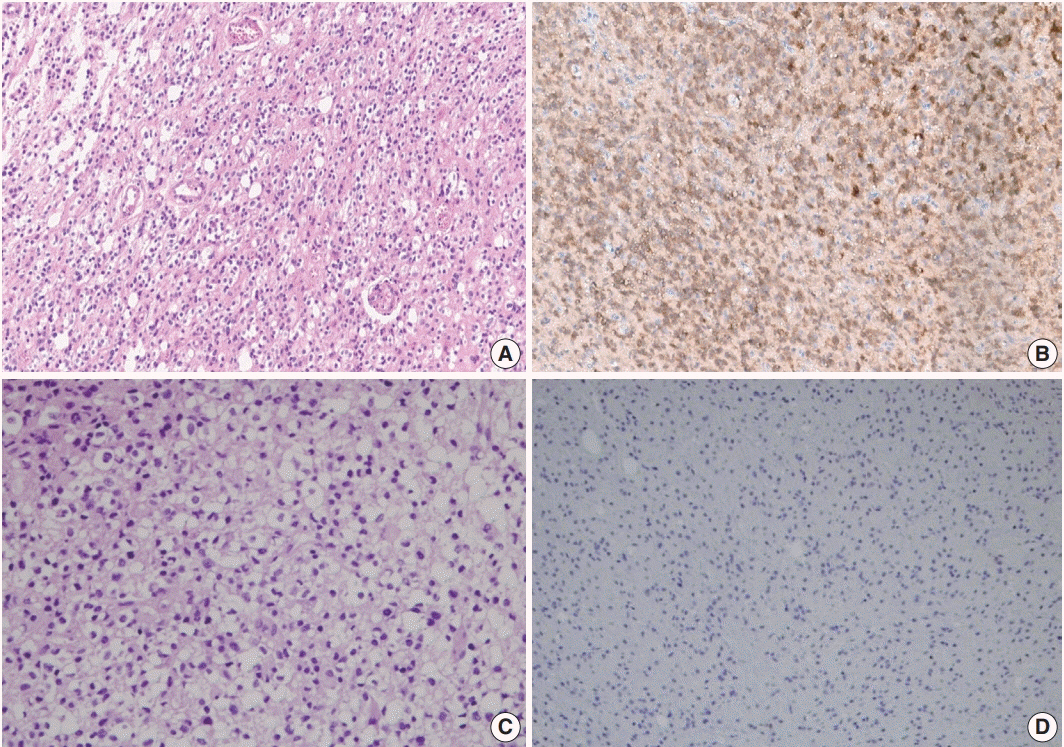
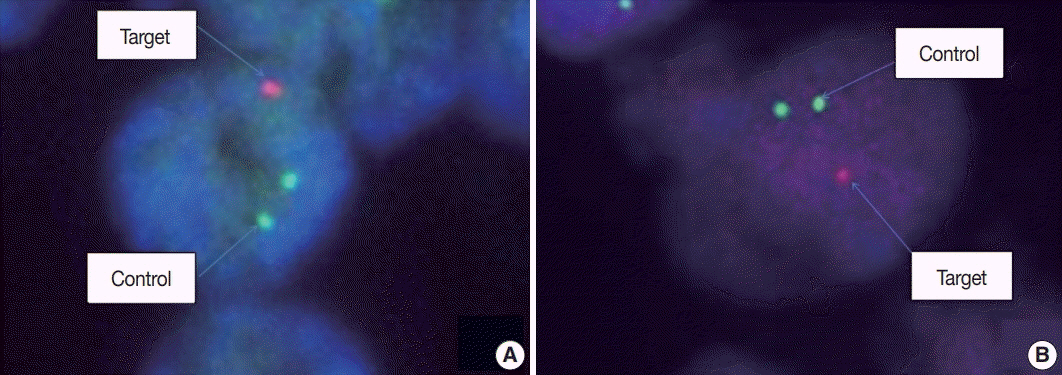
A total of 124 cases of diffuse gliomas were collected, and tissue microarray blocks were made. IDH1 mutation was tested using immunohistochemistry, and 1p/19q co-deletion status was examined using fluorescence in situ hybridization analysis.
RESULTS
According to the 2016 WHO classification, 124 cases of diffuse brain glioma were reclassified as follows: 10 oligodendroglioma, IDH
Keyword
MeSH Terms
-
Astrocytoma
Brain
Central Nervous System
Chromosome Deletion
Classification
Diagnosis
Fluorescence
Glioblastoma
Glioma*
Global Health*
Humans
Immunohistochemistry
In Situ Hybridization
Isocitrate Dehydrogenase
Nervous System Neoplasms*
Nervous System*
Oligodendroglioma
World Health Organization*
Isocitrate Dehydrogenase
Figure
Reference
-
1. Cancer Genome Atlas Research Network, Brat DJ, Verhaak RG, et al. Comprehensive, integrative genomic analysis of diffuse lowergrade gliomas. N Engl J Med. 2015; 372:2481–98.
Article2. Reuss DE, Kratz A, Sahm F, et al. Adult IDH wild type astrocytomas biologically and clinically resolve into other tumor entities. Acta Neuropathol. 2015; 130:407–17.
Article3. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007; 114:97–109.
Article4. Hirose Y, Sasaki H, Abe M, et al. Subgrouping of gliomas on the basis of genetic profiles. Brain Tumor Pathol. 2013; 30:203–8.
Article5. Tabouret E, Nguyen AT, Dehais C, et al. Prognostic impact of the 2016 WHO classification of diffuse gliomas in the French POLA cohort. Acta Neuropathol. 2016; 132:625–34.
Article6. Hainfellner J, Louis DN, Perry A, Wesseling P. Letter in response to David N. Louis et al, International Society of Neuropathology Haarlem Consensus Guidelines for Nervous System Tumor Classification and Grading, Brain Pathology, doi: 10.1111/bpa.12171. Brain Pathol. 2014; 24:671–2.7. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016; 131:803–20.
Article8. Gupta A, Dwivedi T. A simplified overview of World Health Organization classification update of central nervous system tumors 2016. J Neurosci Rural Pract. 2017; 8:629–41.
Article9. Wesseling P, Capper D. WHO 2016 classification of gliomas. Neuropathol Appl Neurobiol. 2018; 44:139–50.
Article10. Akagi Y, Yoshimoto K, Hata N, et al. Reclassification of 400 consecutive glioma cases based on the revised 2016WHO classification. Brain Tumor Pathol. 2018; 35:81–9.
Article11. Iuchi T, Sugiyama T, Ohira M, et al. Clinical significance of the 2016 WHO classification in Japanese patients with gliomas. Brain Tumor Pathol. 2018; 35:71–80.
Article12. Pisapia DJ. The updated World Health Organization glioma classification: cellular and molecular origins of adult infiltrating gliomas. Arch Pathol Lab Med. 2017; 141:1633–45.
Article13. Louis DN, Ellison DW, Brat DJ, et al. cIMPACT-NOW: a practical summary of diagnostic points from Round 1 updates. Brain Pathol. 2019; 29:469–72.
Article14. Boulay JL, Stiefel U, Taylor E, Dolder B, Merlo A, Hirth F. Loss of heterozygosity of TRIM3 in malignant gliomas. BMC Cancer. 2009; 9:71.
Article15. Hata N, Yoshimoto K, Yokoyama N, et al. Allelic losses of chromosome 10 in glioma tissues detected by quantitative single-strand conformation polymorphism analysis. Clin Chem. 2006; 52:370–8.
Article16. Mizoguchi M, Yoshimoto K, Ma X, et al. Molecular characteristics of glioblastoma with 1p/19q co-deletion. Brain Tumor Pathol. 2012; 29:148–53.
Article17. Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008; 116:597–602.18. Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009; 118:469–74.19. Ichimura K, Pearson DM, Kocialkowski S, et al. IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro Oncol. 2009; 11:341–7.20. Sanson M, Marie Y, Paris S, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009; 27:4150–4.
Article21. Mellai M, Piazzi A, Caldera V, et al. IDH1 and IDH2 mutations, immunohistochemistry and associations in a series of brain tumors. J Neurooncol. 2011; 105:345–57.22. von Deimling A, Korshunov A, Hartmann C. The next generation of glioma biomarkers: MGMT methylation, BRAF fusions and IDH1 mutations. Brain Pathol. 2011; 21:74–87.23. Bigner SH, Matthews MR, Rasheed BK, et al. Molecular genetic aspects of oligodendrogliomas including analysis by comparative genomic hybridization. Am J Pathol. 1999; 155:375–86.
Article24. Cohen AL, Holmen SL, Colman H. IDH1 and IDH2 mutations in gliomas. Curr Neurol Neurosci Rep. 2013; 13:345.25. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009; 360:765–73.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Classification and Diagnosis of Adult Glioma: A Scoping Review
- Diffuse Midline Gliomas Harboring the H3 K27M-Mutation in the Bilateral Thalamus and Midbrain: A Case Report and a Review of the Literature
- Major Changes to the 2017 Revision of the World Health Organization Classification of Myeloproliferative Neoplasms
- Adjunctive markers for classification and diagnosis of central nervous system tumors: results of a multi-center neuropathological survey in Korea
- Early High-Grade Transformation of IDH-Mutant Central Nervous System WHO Grade 2 Astrocytoma: A Case Report