Lab Anim Res.
2018 Dec;34(4):302-310. 10.5625/lar.2018.34.4.302.
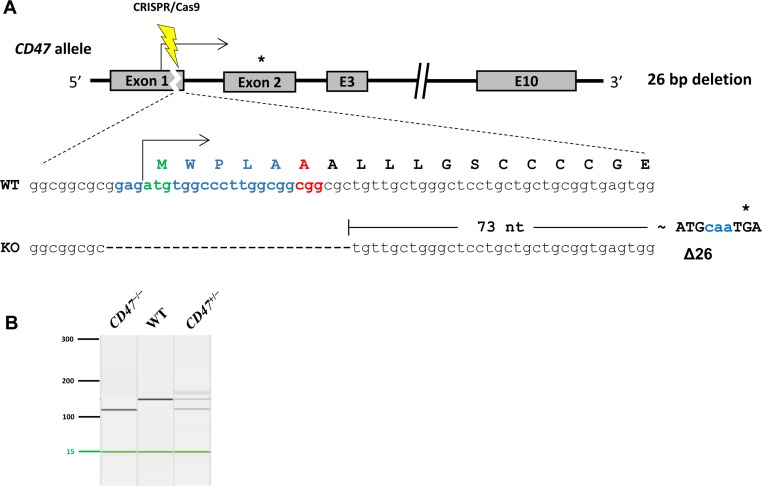
CRISPR/Cas9-mediated knockout of CD47 causes hemolytic anemia with splenomegaly in C57BL/6 mice
- Affiliations
-
- 1Graduate School of Translational Medicine, Seoul National University College of Medicine, Seoul, Korea. bckang@snu.ac.kr
- 2Department of Experimental Animal Research, Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea.
- 3Department of Pathology, Dongguk University Ilsan Hospital, Goyang, Korea.
- 4College of Medicine Severance Biomedical Science Institute, Yonsei University, Seoul, Korea.
- 5Department of Biochemistry, Yonsei University, Seoul, Korea.
- 6Biomedical Center for Animal Resource and Development, Seoul National University, College of Medicine, Seoul, Korea.
- 7Designed Animal and Transplantation Research Institute, Institute of Green Bio Science Technology, Seoul National University, Pyeongchang-gun, Korea.
- KMID: 2459309
- DOI: http://doi.org/10.5625/lar.2018.34.4.302
Abstract
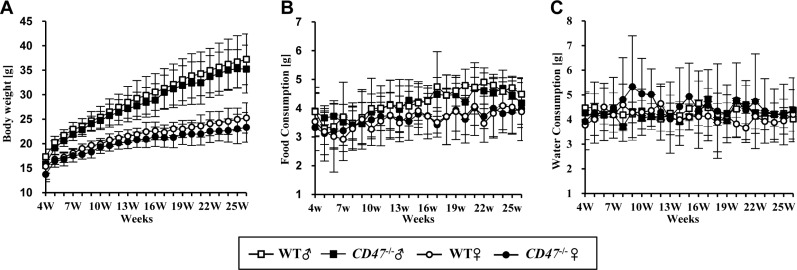
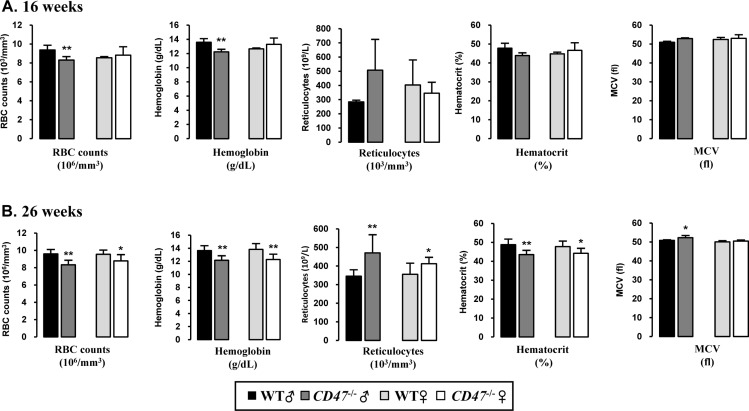
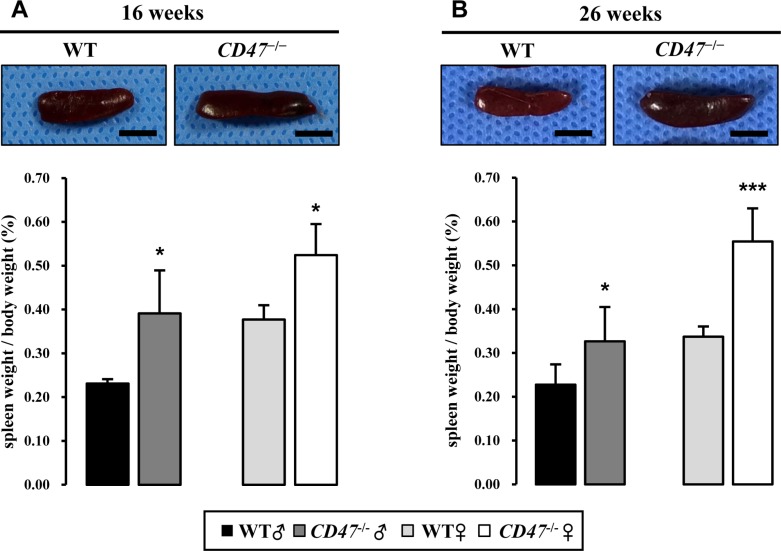
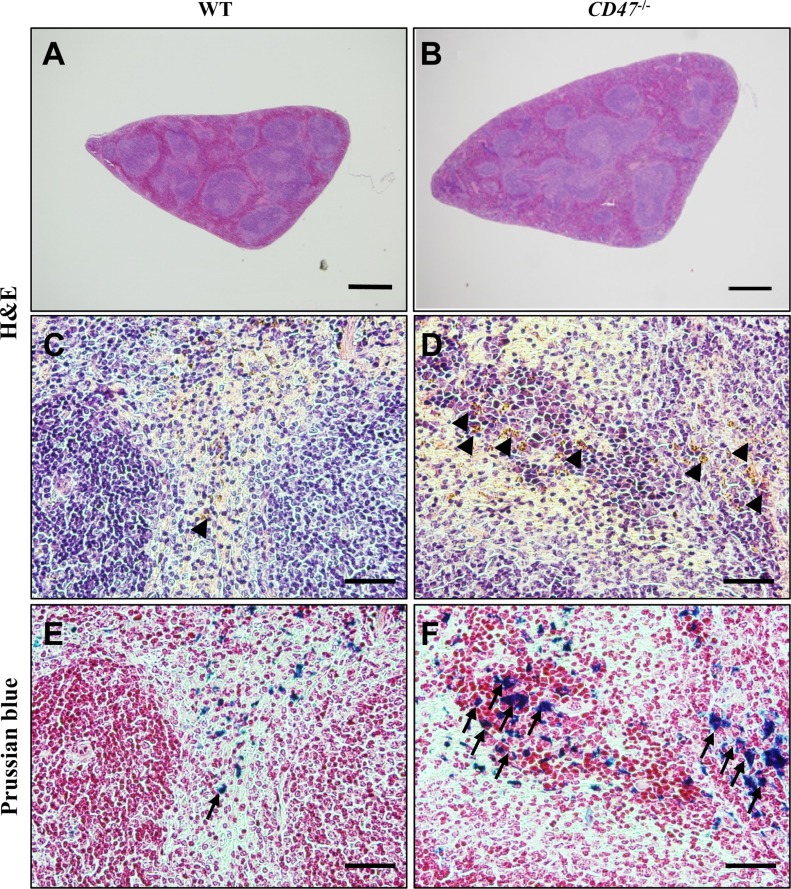
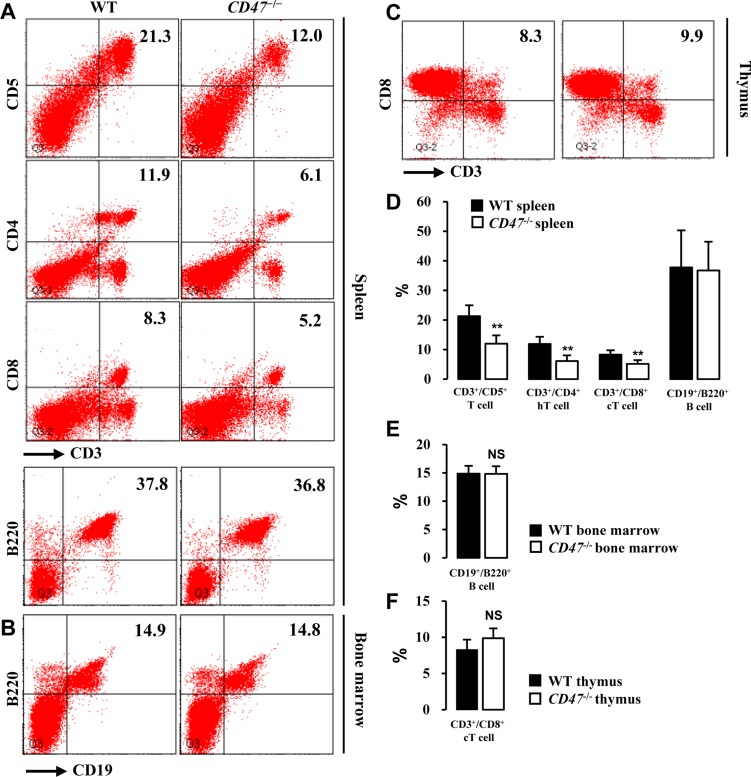
- CD47 (integrin-associated protein), a multi-spanning transmembrane protein expressed in all cells including red blood cells (RBCs) and leukocytes, interacts with signal regulatory protein α (SIRPα) on macrophages and thereby inhibits phagocytosis of RBCs. Recently, we generated a novel C57BL/6J CD47 knockout (CD47(−/−) hereafter) mouse line by employing a CRISPR/Cas9 system at Center for Mouse Models of Human Disease, and here report their hematological phenotypes. On monitoring their birth and development, CD47(−/−) mice were born viable with a natural male-to-female sex ratio and normally developed from birth through puberty to adulthood without noticeable changes in growth, food/water intake compared to their age and sex-matched wild-type littermates up to 26 weeks. Hematological analysis revealed a mild but significant reduction of RBC counts and hemoglobin in 16 week-old male CD47(−/−) mice which were aggravated at the age of 26 weeks with increased reticulocyte counts and mean corpuscular volume (MCV), suggesting hemolytic anemia. Interestingly, anemia in female CD47(−/−) mice became evident at 26 weeks, but splenomegaly was identified in both genders of CD47(−/−) mice from the age of 16 weeks, consistent with development of hemolytic anemia. Additionally, helper and cytotoxic T cell populations were considerably reduced in the spleen, but not in thymus, of CD47(−/−) mice, suggesting a crucial role of CD47 in proliferation of T cells. Collectively, these findings indicate that our CD47(−/−) mice have progressive hemolytic anemia and splenic depletion of mature T cell populations and therefore may be useful as an in vivo model to study the function of CD47.
Keyword
MeSH Terms
Figure
Reference
-
1. Lindberg FP, Lublin DM, Telen MJ, Veile RA, Miller YE, Donis-Keller H, Brown EJ. Rh-related antigen CD47 is the signal-transducer integrin-associated protein. J Biol Chem. 1994; 269(3):1567–1570. PMID: 8294396.
Article2. Reinhold MI, Lindberg FP, Plas D, Reynolds S, Peters MG, Brown EJ. In vivo expression of alternatively spliced forms of integrin-associated protein (CD47). J Cell Sci. 1995; 108(11):3419–3425. PMID: 8586654.
Article3. Lindberg FP, Bullard DC, Caver TE, Gresham HD, Beaudet AL, Brown EJ. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science. 1996; 274(5288):795–798. PMID: 8864123.
Article4. Oldenborg PA, Zheleznyak A, Fang Y-F, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000; 288(5473):2051–2054. PMID: 10856220.
Article5. Armant M, Avice M-N, Hermann P, Rubio M, Kiniwa M, Delespesse G, Sarfati M. CD47 ligation selectively downregulates human interleukin 12 production. J Exp Med. 1999; 190(8):1175–1182. PMID: 10523615.
Article6. Demeure CE, Tanaka H, Mateo V, Rubio M, Delespesse G, Sarfati M. CD47 engagement inhibits cytokine production and maturation of human dendritic cells. J Immunol. 2000; 164(4):2193–2199. PMID: 10657674.
Article7. Mateo V, Lagneaux L, Bron D, Biron G, Armant M, Delespesse G, Sarfati M. CD47 ligation induces caspase-independent cell death in chronic lymphocytic leukemia. Nat Med. 1999; 5(11):1277–1284. PMID: 10545994.
Article8. Waclavicek M, Majdic O, Stulnig T, Berger M, Baumruker T, Knapp W, Pickl WF. T cell stimulation via CD47: agonistic and antagonistic effects of CD47 monoclonal antibody 1/1A4. J Immunol. 1997; 159(11):5345–5354. PMID: 9548474.9. Lavender KJ, Pang WW, Messer RJ, Duley AK, Race B, Phillips K, Scott D, Peterson KE, Chan CK, Dittmer U, Dudek T, Allen TM, Weissman IL, Hasenkrug KJ. BLT-humanized C57BL/6 Rag2−/−γc−/−CD47−/− mice are resistant to GVHD and develop B- and T-cell immunity to HIV infection. Blood. 2013; 122(25):4013–4020. PMID: 24021673.
Article10. Lavender KJ, Messer RJ, Race B, Hasenkrug KJ. Production of bone marrow, liver, thymus (BLT) humanized mice on the C57BL/6 Rag2(−/−)γc(−/−)CD47(−/−) background. J Immunol Methods. 2014; 407:127–134. PMID: 24769067.
Article11. VAN PUTTEN LM, Croon F. The life span of red cells in the rat and the mouse as determined by labeling with DFP32 in vivo. Blood. 1958; 13(8):789–794. PMID: 13560578.
Article12. Oldenborg PA, Gresham HD, Chen Y, Izui S, Lindberg FP. Lethal autoimmune hemolytic anemia in CD47-deficient nonobese diabetic (NOD) mice. Blood. 2002; 99(10):3500–3504. PMID: 11986200.
Article13. Lee JH, Park JH, Nam TW, Seo SM, Kim JY, Lee HK, Han JH, Park SY, Choi YK, Lee HW. Differences between immunodeficient mice generated by classical gene targeting and CRISPR/Cas9-mediated gene knockout. Transgenic Res. 2018; 27(3):241–251. PMID: 29594927.
Article14. Council NR. Guide for the care and use of laboratory animals. National Academies Press;2010.15. Kim JI, Park JS, Kim H, Ryu SK, Kwak J, Kwon E, Yun JW, Nam KT, Lee HW, Kang BC. CRISPR/Cas9-mediated knockout of Rag-2 causes systemic lymphopenia with hypoplastic lymphoid organs in FVB mice. Lab Anim Res. 2018; 34(4):166–175.
Article16. Wennberg E, Weiss L. Splenomegaly and hemolytic anemia induced in rats by methylcellulose-an electron microscopic study. J Morphol. 1967; 122(1):35–61. PMID: 6034783.17. Wagner KU, Claudio E, Rucker EB 3rd, Riedlinger G, Broussard C, Schwartzberg PL, Siebenlist U, Hennighausen L. Conditional deletion of the Bcl-x gene from erythroid cells results in hemolytic anemia and profound splenomegaly. Development. 2000; 127(22):4949–4958. PMID: 11044408.
Article18. Sato-Hashimoto M, Saito Y, Ohnishi H, Iwamura H, Kanazawa Y, Kaneko T, Kusakari S, Kotani T, Mori M, Murata Y, Okazawa H, Ware CF, Oldenborg PA, Nojima Y, Matozaki T. Signal regulatory protein α regulates the homeostasis of T lymphocytes in the spleen. J Immunol. 2011; 187(1):291–297. PMID: 21632712.
Article19. Guimont-Desrochers F, Beauchamp C, Chabot-Roy G, Dugas V, Hillhouse EE, Dusseault J, Langlois G, Gautier-Ethier P, Darwiche J, Sarfati M, Lesage S. Absence of CD47 in vivo influences thymic dendritic cell subset proportions but not negative selection of thymocytes. Int Immunol. 2009; 21(2):167–177. PMID: 19147837.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CRISPR-Cas9 system in autosomal dominant polycystic kidney disease: a comprehensive review
- Generation of knockout mouse models of cyclin-dependent kinase inhibitors by engineered nuclease-mediated genome editing
- The length of guide RNA and target DNA heteroduplex effects on CRISPR/Cas9 mediated genome editing efficiency in porcine cells
- CRISPR/Cas9-mediated generation of a Plac8 knockout mouse model
- Genome editing: the road of CRISPR/Cas9 from bench to clinic