Nutr Res Pract.
2019 Jun;13(3):205-213. 10.4162/nrp.2019.13.3.205.
Supplementation with psyllium seed husk reduces myocardial damage in a rat model of ischemia/reperfusion
- Affiliations
-
- 1Department of Biochemistry, School of Medicine, Catholic University of Daegu, 33 Duryugongwon-ro 17-gil, Nam-gu, Daegu 42472, Republic of Korea. leejw@cu.ac.kr
- KMID: 2453284
- DOI: http://doi.org/10.4162/nrp.2019.13.3.205
Abstract
- BACKGROUND/OBJECTIVES
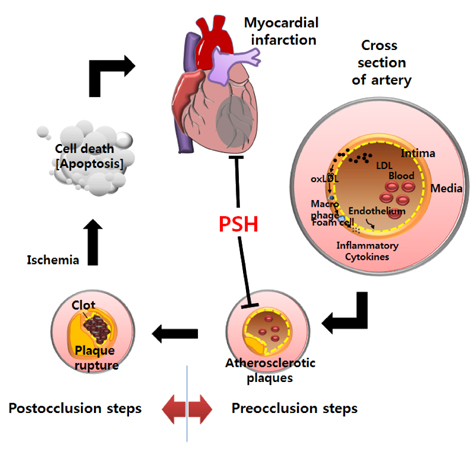
Myocardial infarction (MI) is caused by extensive myocardial damage attributed to the occlusion of coronary arteries. Our previous study in a rat model of ischemia/reperfusion (I/R) demonstrated that administration of arabinoxylan (AX), comprising arabinose and xylose, protects against myocardial injury. In this study, we undertook to investigate whether psyllium seed husk (PSH), a safe dietary fiber containing a high level of AX (> 50%), also imparts protection against myocardial injury in the same rat model.
MATERIALS/METHODS
Rats were fed diets supplemented with PSH (1, 10, or 100 mg/kg/d) for 3 d. The rats were then subjected to 30 min ischemia through ligation of the left anterior descending coronary artery, followed by 3 h reperfusion through release of the ligation. The hearts were harvested and cut into four slices. To assess infarct size (IS), an index representing heart damage, the slices were stained with 2,3,5-triphenyltetrazolium chloride (TTC). To elucidate underlying mechanisms, Western blotting was performed for the slices.
RESULTS
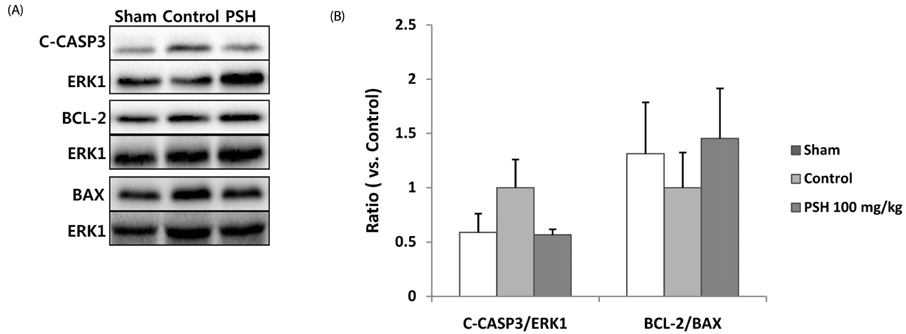
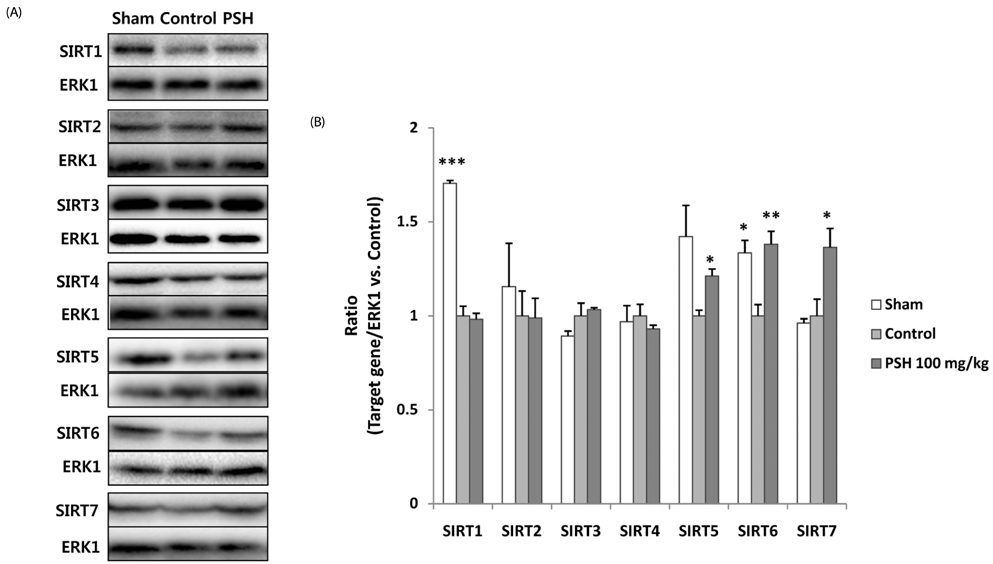
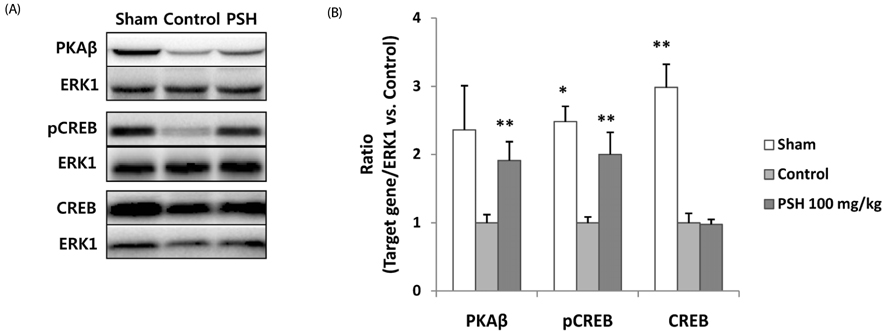
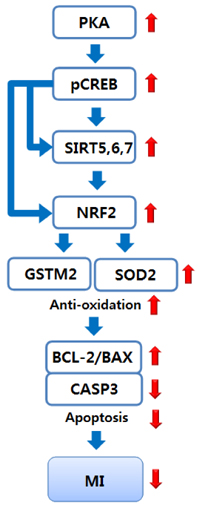
Supplementation with 10 or 100 mg/kg/d of PSH significantly reduces the IS. PSH supplementation (100 mg/kg/d) tends to reduce caspase-3 generation and increase BCL-2/BAX ratio. PSH supplementation also upregulates the expression of nuclear factor erythroid 2-related factor 2 (NRF2), and its target genes including antioxidant enzymes such as glutathione S-transferase mu 2 (GSTM2) and superoxide dismutase 2 (SOD2). PSH supplementation upregulates some sirtuins (NAD+-dependent deacetylases) including SIRT5 (a mitochondrial sirtuin) and SIRT6 and SIRT7 (nuclear sirtuins). Finally, PSH supplementation upregulates the expression of protein kinase A (PKA), and increases phosphorylated cAMP response element-binding protein (CREB) (pCREB), a target protein of PKA.
CONCLUSIONS
The results from this study indicate that PSH consumption reduces myocardial I/R injury in rats by inhibiting the apoptotic cascades through modulation of gene expression of several genes located upstream of apoptosis. Therefore, we believe that PSH can be developed as a functional food that would be beneficial in the prevention of MI.
Keyword
MeSH Terms
-
Animals
Apoptosis
Arabinose
Blotting, Western
Caspase 3
Coronary Vessels
Cyclic AMP Response Element-Binding Protein
Cyclic AMP-Dependent Protein Kinases
Diet
Dietary Fiber
Functional Food
Gene Expression
Glutathione Transferase
Heart
Infarction
Ischemia
Ligation
Models, Animal*
Myocardial Infarction
Psyllium*
Rats*
Reperfusion
Sirtuins
Superoxide Dismutase
Xylose
Arabinose
Caspase 3
Cyclic AMP Response Element-Binding Protein
Cyclic AMP-Dependent Protein Kinases
Glutathione Transferase
Psyllium
Sirtuins
Superoxide Dismutase
Xylose
Figure
Reference
-
1. Lim SH, Lee J. Xyloglucan intake attenuates myocardial injury by inhibiting apoptosis and improving energy metabolism in a rat model of myocardial infarction. Nutr Res. 2017; 45:19–29.
Article2. Lim SH. Larch arabinogalactan attenuates myocardial injury by inhibiting apoptotic cascades in a rat model of ischemia-reperfusion. J Med Food. 2017; 20:691–699.
Article3. Kim MY, Lim SH, Lee J. Intake of hot water-extracted apple protects against myocardial injury by inhibiting apoptosis in an ischemia/reperfusion rat model. Nutr Res. 2014; 34:951–960.
Article4. Lim SH, Kim MJ, Han MJ, Kim Y, Lee J. Prevention of ischemic diseases and cognitive disorders through wheat consumption. In : Duncan LT, editor. Advances in Health and Disease. Hauppauge (NY): Nova Science Publishers, Inc.;2018. p. 1–66.5. Soler EP, Ruiz VC. Epidemiology and risk factors of cerebral ischemia and ischemic heart diseases: similarities and differences. Curr Cardiol Rev. 2010; 6:138–149.
Article6. Hajar R. Risk factors for coronary artery disease: historical perspectives. Heart Views. 2017; 18:109–114.
Article7. Hurtubise J, McLellan K, Durr K, Onasanya O, Nwabuko D, Ndisang JF. The different facets of dyslipidemia and hypertension in atherosclerosis. Curr Atheroscler Rep. 2016; 18:82.
Article8. Park KH, Park WJ. Endothelial dysfunction: clinical implications in cardiovascular disease and therapeutic approaches. J Korean Med Sci. 2015; 30:1213–1225.
Article9. Maiolino G, Rossitto G, Caielli P, Bisogni V, Rossi GP, Calò LA. The role of oxidized low-density lipoproteins in atherosclerosis: the myths and the facts. Mediators Inflamm. 2013; 2013:714653.
Article10. Lim SH, Kim MY, Lee J. Apple pectin, a dietary fiber, ameliorates myocardial injury by inhibiting apoptosis in a rat model of ischemia/reperfusion. Nutr Res Pract. 2014; 8:391–397.
Article11. Wei ZH, Wang H, Chen XY, Wang BS, Rong ZX, Wang BS, Su BH, Chen HZ. Time- and dose-dependent effect of psyllium on serum lipids in mild-to-moderate hypercholesterolemia: a meta-analysis of controlled clinical trials. Eur J Clin Nutr. 2009; 63:821–827.
Article12. Ribas SA, Cunha DB, Sichieri R, Santana da Silva LC. Effects of psyllium on LDL-cholesterol concentrations in Brazilian children and adolescents: a randomised, placebo-controlled, parallel clinical trial. Br J Nutr. 2015; 113:134–141.
Article13. Xing LC, Santhi D, Shar AG, Saeed M, Arain MA, Shar AH, Bhutto ZA, Katar MU, Manzoor R, El-Hack ME, Alagawany M, Dhama K, Ling MC. Psyllium husk (Plantago ovata) as a potent hypocholesterolemic agent in animal, human and poultry. Int J Pharmacol. 2017; 13:690–697.
Article14. Gibb RD, McRorie JW Jr, Russell DA, Hasselblad V, D'Alessio DA. Psyllium fiber improves glycemic control proportional to loss of glycemic control: a meta-analysis of data in euglycemic subjects, patients at risk of type 2 diabetes mellitus, and patients being treated for type 2 diabetes mellitus. Am J Clin Nutr. 2015; 102:1604–1614.
Article15. Khan K, Jovanovski E, Ho HV, Marques AC, Zurbau A, Mejia SB, Sievenpiper JL, Vuksan V. The effect of viscous soluble fiber on blood pressure: a systematic review and meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2018; 28:3–13.
Article16. Obata K, Ikeda K, Yamasaki M, Yamori Y. Dietary fiber, psyllium, attenuates salt-accelerated hypertension in stroke-prone spontaneously hypertensive rats. J Hypertens. 1998; 16:1959–1964.
Article17. Raedschelders K, Ansley DM, Chen DD. The cellular and molecular origin of reactive oxygen species generation during myocardial ischemia and reperfusion. Pharmacol Ther. 2012; 133:230–255.
Article18. Zhang Y, Martin SG. Redox proteins and radiotherapy. Clin Oncol (R Coll Radiol). 2014; 26:289–300.
Article19. Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014; 2014:360438.
Article20. Zhou S, Sun W, Zhang Z, Zheng Y. The role of Nrf2-mediated pathway in cardiac remodeling and heart failure. Oxid Med Cell Longev. 2014; 2014:260429.
Article21. Murphy KE, Park JJ. Can co-activation of Nrf2 and neurotrophic signaling pathway slow Alzheimer's disease. Int J Mol Sci. 2017; 18:1168.
Article22. Matsushima S, Sadoshima J. The role of sirtuins in cardiac disease. Am J Physiol Heart Circ Physiol. 2015; 309:H1375–H1389.
Article23. Yu W, Xu M, Zhang T, Zhang Q, Zou C. Mst1 promotes cardiac ischemia-reperfusion injury by inhibiting the ERK-CREB pathway and repressing FUNDC1-mediated mitophagy. J Physiol Sci. 2019; 69:113–127.
Article24. Zhang Y, Wang XL, Zhao J, Wang YJ, Lau WB, Yuan YX, Gao EH, Koch WJ, Ma XL. Adiponectin inhibits oxidative/nitrative stress during myocardial ischemia and reperfusion via PKA signaling. Am J Physiol Endocrinol Metab. 2013; 305:E1436–E1443.
Article25. Arai AE. Healing after myocardial infarction: a loosely defined process. JACC Cardiovasc Imaging. 2015; 8:680–683.26. Lim SH, Kim Y, Yun KN, Kim JY, Jang JH, Han MJ, Lee J. Plant-based foods containing cell wall polysaccharides rich in specific active monosaccharides protect against myocardial injury in rat myocardial infarction models. Sci Rep. 2016; 6:38728.
Article27. Van Craeyveld V, Delcour JA, Courtin CM. Ball milling improves extractability and affects molecular properties of psyllium (Plantago ovata Forsk) seed husk arabinoxylan. J Agric Food Chem. 2008; 56:11306–11311.
Article28. Lim SH, Kim MJ, Lee J. Intake of psyllium seed husk reduces white matter damage in a rat model of chronic hypoperfusion. Nutr Res. 2019; DOI: 10.1016/j.nutres.2019.04.002.29. Lim SH, Lee J. Protection of the brain through supplementation with larch arabinogalactan in a rat model of vascular dementia. Nutr Res Pract. 2017; 11:381–387.
Article30. Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999; 6:99–104.
Article31. Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008; 9:47–59.
Article32. Edlich F. BCL-2 proteins and apoptosis: recent insights and unknowns. Biochem Biophys Res Commun. 2018; 500:26–34.
Article33. Sands WA, Palmer TM. Regulating gene transcription in response to cyclic AMP elevation. Cell Signal. 2008; 20:460–466.
Article34. Zhao D, Feng P, Sun Y, Qin Z, Zhang Z, Tan Y, Gao E, Lau WB, Ma X, Yang J, Yu S, Xu X, Yi D, Yi W. Cardiac-derived CTRP9 protects against myocardial ischemia/reperfusion injury via calreticulin-dependent inhibition of apoptosis. Cell Death Dis. 2018; 9:723.
Article35. Wang XX, Wang XL, Tong MM, Gan L, Chen H, Wu SS, Chen JX, Li RL, Wu Y, Zhang HY, Zhu Y, Li YX, He JH, Wang M, Jiang W. SIRT6 protects cardiomyocytes against ischemia/reperfusion injury by augmenting FoxO3α-dependent antioxidant defense mechanisms. Basic Res Cardiol. 2016; 111:13.
Article36. Araki S, Izumiya Y, Rokutanda T, Ianni A, Hanatani S, Kimura Y, Onoue Y, Senokuchi T, Yoshizawa T, Yasuda O, Koitabashi N, Kurabayashi M, Braun T, Bober E, Yamagata K, Ogawa H. Sirt7 contributes to myocardial tissue repair by maintaining transforming growth factor-β signaling pathway. Circulation. 2015; 132:1081–1093.
Article37. Liu B, Che W, Zheng C, Liu W, Wen J, Fu H, Tang K, Zhang J, Xu Y. SIRT5: a safeguard against oxidative stress-induced apoptosis in cardiomyocytes. Cell Physiol Biochem. 2013; 32:1050–1059.
Article38. Fishilevich S, Nudel R, Rappaport N, Hadar R, Plaschkes I, Iny Stein T, Rosen N, Kohn A, Twik M, Safran M, Lancet D, Cohen D. GeneHancer: genome-wide integration of enhancers and target genes in GeneCards. Database (Oxford). 2017; 2017:1.
Article39. Pan H, Guan D, Liu X, Li J, Wang L, Wu J, Zhou J, Zhang W, Ren R, Zhang W, Li Y, Yang J, Hao Y, Yuan T, Yuan G, Wang H, Ju Z, Mao Z, Li J, Qu J, Tang F, Liu GH. SIRT6 safeguards human mesenchymal stem cells from oxidative stress by coactivating NRF2. Cell Res. 2016; 26:190–205.
Article40. Zhang W, Wei R, Zhang L, Tan Y, Qian C. Sirtuin 6 protects the brain from cerebral ischemia/reperfusion injury through NRF2 activation. Neuroscience. 2017; 366:95–104.
Article41. Liao CY, Kennedy BK. SIRT6, oxidative stress, and aging. Cell Res. 2016; 26:143–144.
Article42. Impey S, McCorkle SR, Cha-Molstad H, Dwyer JM, Yochum GS, Boss JM, McWeeney S, Dunn JJ, Mandel G, Goodman RH. Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions. Cell. 2004; 119:1041–1054.
Article43. Jenkins DJ, Kendall CW, Vuksan V, Vidgen E, Parker T, Faulkner D, Mehling CC, Garsetti M, Testolin G, Cunnane SC, Ryan MA, Corey PN. Soluble fiber intake at a dose approved by the US Food and Drug Administration for a claim of health benefits: serum lipid risk factors for cardiovascular disease assessed in a randomized controlled crossover trial. Am J Clin Nutr. 2002; 75:834–839.
Article44. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016; 7:27–31.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Psyllium Husk for the Management of Neurogenic Bowel in Chronic Spinal Cord Injured Persons
- Effects of Ivabradine on Left Ventricular Systolic Function and Cardiac Fibrosis in Rat Myocardial Ischemia-Reperfusion Model
- Organ free radical induced damage after ischemia and reperfusion in rat kidneys
- Cardiodynamics and Infarct Size in Regional and Global Ischemic Isolated Heart Model: Comparison of 1 Hour and 2 Hours Reperfusion
- Myocardial protective effect by ulinastatin via an anti-inflammatory response after regional ischemia/reperfusion injury in an in vivo rat heart model