Korean Circ J.
2012 Sep;42(9):600-605. 10.4070/kcj.2012.42.9.600.
Cardiodynamics and Infarct Size in Regional and Global Ischemic Isolated Heart Model: Comparison of 1 Hour and 2 Hours Reperfusion
- Affiliations
-
- 1Institute of Cardiovascular Research Center, Pusan National University Yangsan Hospital, Yangsan, Korea. weonjo@pnuyh.co.kr
- 2Institute of Medical Science, Keimyung University, Daegu, Korea.
- 3Department of Anesthesiology, University of North Carolina, Chapel Hill, NC, USA.
- KMID: 2297890
- DOI: http://doi.org/10.4070/kcj.2012.42.9.600
Abstract
- BACKGROUND AND OBJECTIVES
We investigated whether 1 hour reperfusion is enough to assess cardiodynamics and infarct size in both regional ischemia (RI) and global ischemia (GI) in isolated rat heart models.
MATERIALS AND METHODS
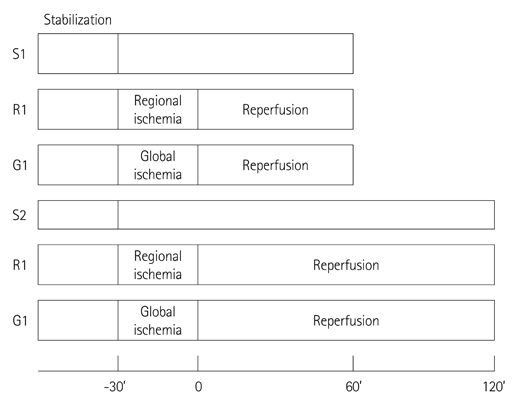
Hearts were randomly assigned to one of the following groups (each n=14): 1) Sham hearts for 1 hour; 2) Sham hearts for 2 hours; 3) 30 minutes RI followed by 1 hour reperfusion; 4) 30 minutes of RI followed by 2 hours reperfusion; 5) 30 minutes GI followed by 1 hour reperfusion; and 6) 30 minutes GI followed by 2 hours reperfusion.
RESULTS
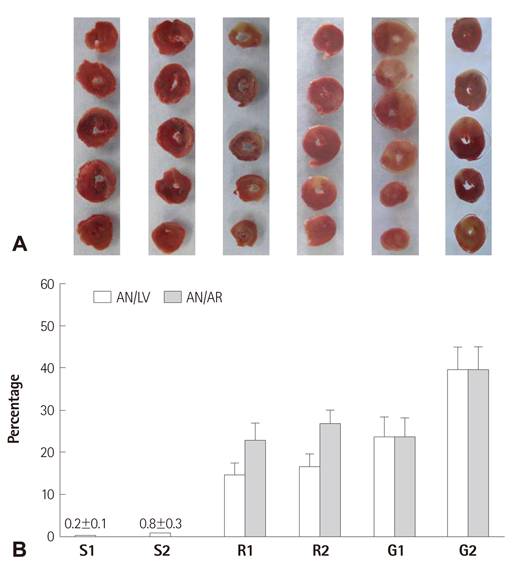
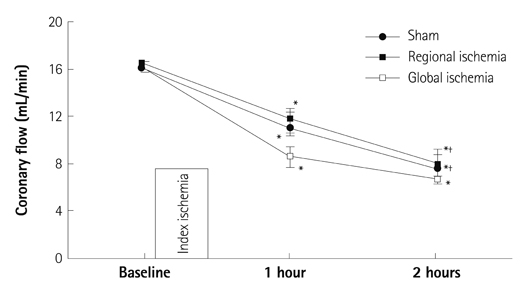
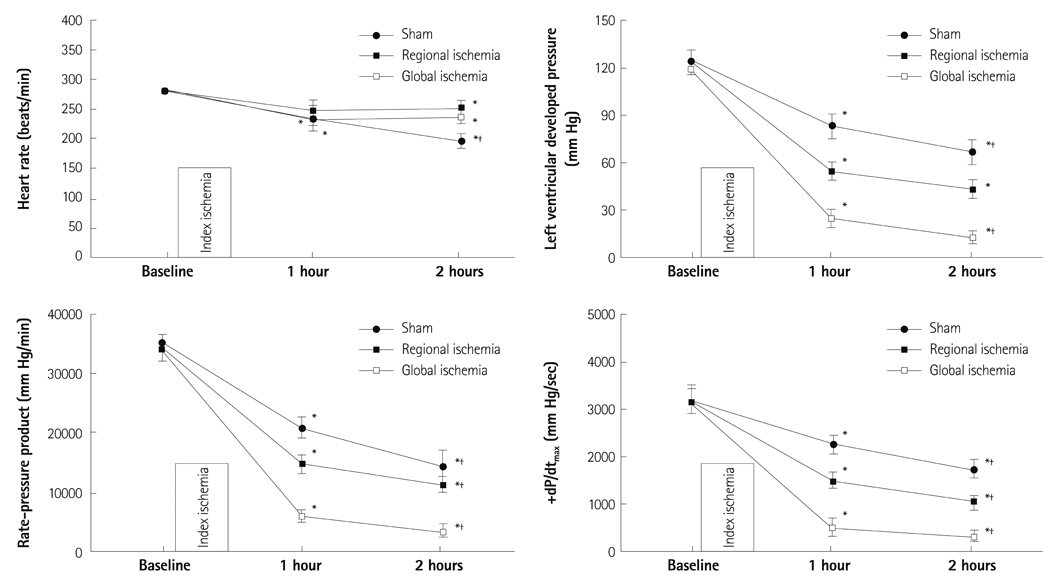
There were no significant differences in infarct size between 1 hour and 2 hours reperfusion in both RI and GI. Left ventricular developed pressure was significantly decreased at both 1 hour and 2 hours reperfusion in groups of RI and GI compared to baseline (p<0.01). Rate-pressure product and +dP/dtmax also significantly decreased compared to baseline level at both 1 hour and 2 hours reperfusion in groups of RI and GI (p<0.05).
CONCLUSION
There was no significant difference in infarct size between 1 hour and 2 hours reperfusion in groups of RI and GI. Cardiodynamic variables measured at 1 hour and 2 hours reperfusion significantly decreased compared to baseline level. Our data suggests that reperfusion of 1 hour is sufficient to assess cardiodynamics in both regional and global ischemic isolated hearts model.
MeSH Terms
Figure
Reference
-
1. Sako EY, Kingsley-Hickman PB, From AH, Ugurbil K, Foker JE. Substrate effects in the post-ischemic myocardium. J Surg Res. 1988. 44:430–435.2. Stark G, Huber U, Hofer E, Tritthart HA. Continuous ECG measurements of intracardiac activity from the surface of Langendorff-perfused guinea pig hearts. Basic Res Cardiol. 1987. 82:437–444.3. Tanguay M, Blaise G, Dumont L, Beique G, Hollmann C. Beneficial effects of volatile anesthetics on decrease in coronary flow and myocardial contractility induced by oxygen-derived free radicals in isolated rabbit hearts. J Cardiovasc Pharmacol. 1991. 18:863–870.4. Batchu SN, Law E, Brocks DR, Falck JR, Seubert JM. Epoxyeicosatrienoic acid prevents postischemic electrocardiogram abnormalities in an isolated heart model. J Mol Cell Cardiol. 2009. 46:67–74.5. Dickson EW, Hogrefe CP, Ludwig PS, Ackermann LW, Stoll LL, Denning GM. Exercise enhances myocardial ischemic tolerance via an opioid receptor-dependent mechanism. Am J Physiol Heart Circ Physiol. 2008. 294:H402–H408.6. Garcia SC, Pomblum V, Gams E, Langenbach MR, Schipke JD. Independency of myocardial stunning of endothelial stunning? Basic Res Cardiol. 2007. 102:359–367.7. Lochner A, Genade S, Moolman JA. Ischemic preconditioning: infarct size is a more reliable endpoint than functional recovery. Basic Res Cardiol. 2003. 98:337–346.8. Ofir M, Arad M, Porat E, et al. Increased glycogen stores due to gamma-AMPK overexpression protects against ischemia and reperfusion damage. Biochem Pharmacol. 2008. 75:1482–1491.9. Gross GJ, Baker JE, Hsu A, Wu HE, Falck JR, Nithipatikom K. Evidence for a role of opioids in epoxyeicosatrienoic acid-induced cardioprotection in rat hearts. Am J Physiol Heart Circ Physiol. 2010. 298:H2201–H2207.10. Lamberts RR, Onderwater G, Hamdani N, et al. Reactive oxygen species-induced stimulation of 5'AMP-activated protein kinase mediates sevoflurane-induced cardioprotection. Circulation. 2009. 120:11 Suppl. S10–S15.11. Xi J, McIntosh R, Shen X, et al. Adenosine A2A and A2B receptors work in concert to induce a strong protection against reperfusion injury in rat hearts. J Mol Cell Cardiol. 2009. 47:684–690.12. Ferrera R, Benhabbouche S, Bopassa JC, Li B, Ovize M. One hour reperfusion is enough to assess function and infarct size with TTC staining in Langendorff rat model. Cardiovasc Drugs Ther. 2009. 23:327–331.13. Jang Y, Xi J, Wang H, Mueller RA, Norfleet EA, Xu Z. Postconditioning prevents reperfusion injury by activating delta-opioid receptors. Anesthesiology. 2008. 108:243–250.14. Song DK, Jang Y, Kim JH, Chun KJ, Lee D, Xu Z. Polyphenol (-)-epigallocatechin gallate during ischemia limits infarct size via mitochondrial K(ATP) channel activation in isolated rat hearts. J Korean Med Sci. 2010. 25:380–386.15. Bouwman RA, Vreden MJ, Hamdani N, et al. Effect of bupivacaine on sevoflurane-induced preconditioning in isolated rat hearts. Eur J Pharmacol. 2010. 647:132–138.16. Taliyan R, Singh M, Sharma PL, Yadav HN, Sidhu KS. Possible involvement of α1-adrenergic receptor and K(ATP) channels in cardioprotective effect of remote aortic preconditioning in isolated rat heart. J Cardiovasc Dis Res. 2010. 1:145–151.17. Lee DS, Steinbaugh GE, Quarrie R, et al. Ischemic postconditioning does not provide cardioprotection from long-term ischemic injury in isolated male or female rat hearts. J Surg Res. 2010. 164:175–181.18. Kolettis TM, Kontaras K, Spartinos I, et al. Dose-dependent effects of sildenafil on post-ischaemic left ventricular function in the rat isolated heart. J Pharm Pharmacol. 2010. 62:346–351.19. Maslov LN, Lasukova OV, Krylatov AV, et al. Role of cannabinoid receptors in the regulation of cardiac contractility during ischemia/reperfusion. Bull Exp Biol Med. 2006. 142:557–561.20. Rohlicek CV, Viau S, Trieu P, Hébert TE. Effects of neonatal hypoxia in the rat on inotropic stimulation of the adult heart. Cardiovasc Res. 2005. 65:861–868.21. Gelpi RJ, Morales C, Cohen MV, Downey JM. Xanthine oxidase contributes to preconditioning's preservation of left ventricular developed pressure in isolated rat heart: developed pressure may not be an appropriate end-point for studies of preconditioning. Basic Res Cardiol. 2002. 97:40–46.22. Chen HT, Yang CX, Li H, et al. Cardioprotection of sevoflurane postconditioning by activating extracellular signal-regulated kinase 1/2 in isolated rat hearts. Acta Pharmacol Sin. 2008. 29:931–941.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of propofol on myocardial protection after regional ischemia-reperfusion injury in an in vivo rat heart model
- Effect of Reperfusion Experimental Myocardial Infarction in Rats
- Effect of Endothelin Antagonists on Myocardial Infarct Size after Coronary Artery Occlusion and Reperfusion in Rat
- Cardioprotective Effect by Preconditioning with Calcium-free Solution
- Dose remifentanil have a myocardial protective effect against a regional ischemia-reperfusion injury in an in vivo rat heart model?