J Korean Neurosurg Soc.
2019 May;62(3):313-320. 10.3340/jkns.2019.0033.
Clinical Pearls and Advances in Molecular Researches of Epilepsy-Associated Tumors
- Affiliations
-
- 1Division of Pediatric Neurosurgery, Seoul National University Children's Hospital, Seoul, Korea. nsthomas@snu.ac.kr
- KMID: 2453236
- DOI: http://doi.org/10.3340/jkns.2019.0033
Abstract
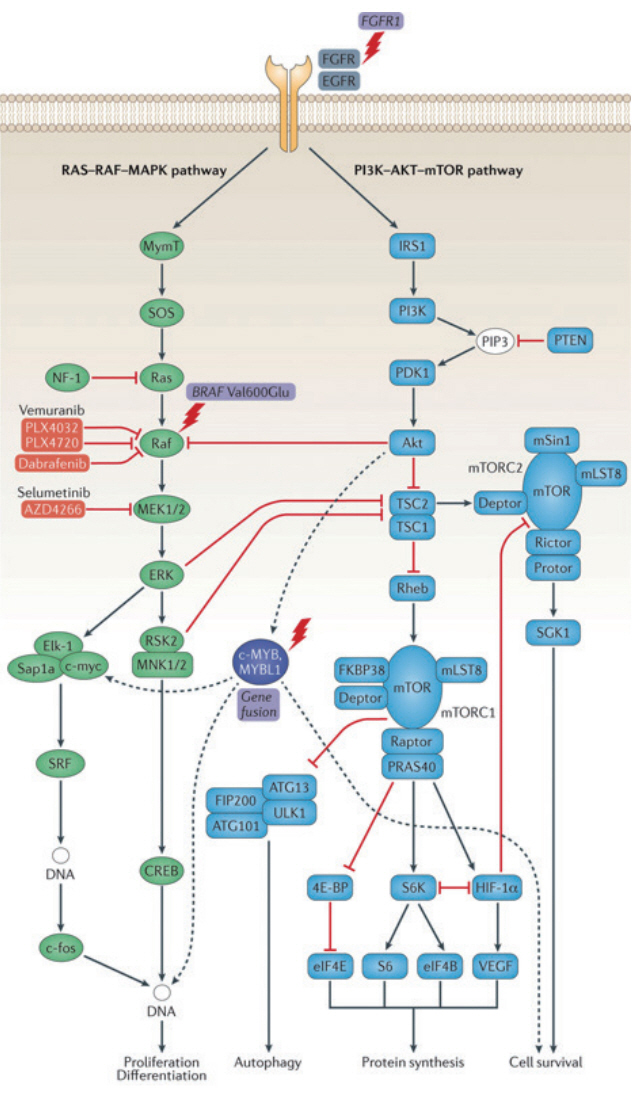
- Brain tumors are the second most common type of structural brain lesion that causes chronic epilepsy. Patients with low-grade brain tumors often experience chronic drug-resistant epilepsy starting in childhood, which led to the concept of long-term epilepsy-associated tumors (LEATs). Dysembryoplastic neuroepithelial tumor and ganglioglioma are representative LEATs and are characterized by young age of onset, frequent temporal lobe location, benign tumor biology, and chronic epilepsy. Although highly relevant in clinical epileptology, the concept of LEATs has been criticized in the neuro-oncology field. Recent genomic and molecular studies have challenged traditional views on LEATs and low-grade gliomas. Molecular studies have revealed that low-grade gliomas can largely be divided into three groups : LEATs, pediatric-type diffuse low-grade glioma (DLGG; astrocytoma and oligodendroglioma), and adult-type DLGG. There is substantial overlap between conventional LEATs and pediatric-type DLGG in regard to clinical features, histology, and molecular characteristics. LEATs and pediatric-type DLGG are characterized by mutations in BRAF, FGFR1, and MYB/MYBL1, which converge on the RAS-RAF-MAPK pathway. Gene (mutation)-centered classification of epilepsy-associated tumors could provide new insight into these heterogeneous and diverse neoplasms and may lead to novel molecular targeted therapies for epilepsy in the near future.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Banan R, Hartmann C. The new WHO 2016 classification of brain tumors-what neurosurgeons need to know. Acta Neurochir (Wien). 159:403–418. 2017.
Article2. Barkovich AJ, Guerrini R, Kuzniecky RI, Jackson GD, Dobyns WB. A developmental and genetic classification for malformations of cortical development: update 2012. Brain. 135(Pt 5):1348–1369. 2012.
Article3. Baumgarten P, Sarlak M, Baumgarten G, Marquardt G, Seifert V, Strzelczyk A, et al. Focused review on seizures caused by meningiomas. Epilepsy Behav. 88:146–151. 2018.
Article4. Blümcke I, Aronica E, Becker A, Capper D, Coras R, Honavar M, et al. Low-grade epilepsy-associated neuroepithelial tumours - the 2016 WHO classification. Nat Rev Neurol. 12:732–740. 2016.
Article5. Blümcke I, Luyken C, Urbach H, Schramm J, Wiestler OD. An isomorphic subtype of long-term epilepsy-associated astrocytomas associated with benign prognosis. Acta Neuropathol. 107:381–388. 2004.
Article6. Blumcke I, Spreafico R, Haaker G, Coras R, Kobow K, Bien CG, et al. Histopathological findings in brain tissue obtained during epilepsy surgery. N Engl J Med. 377:1648–1656. 2017.
Article7. Bonney PA, Boettcher LB, Conner AK, Glenn CA, Briggs RG, Santucci JA, et al. Review of seizure outcomes after surgical resection of dysembryoplastic neuroepithelial tumors. J Neurooncol. 126:1–10. 2016.
Article8. Chappé C, Padovani L, Scavarda D, Forest F, Nanni-Metellus I, Loundou A, et al. Dysembryoplastic neuroepithelial tumors share with pleomorphic xanthoastrocytomas and gangliogliomas BRAF(V600E) mutation and expression. Brain Pathol. 23:574–583. 2013.
Article9. Chassoux F, Daumas-Duport C. Dysembryoplastic neuroepithelial tumors: where are we now? Epilepsia 54 Suppl. 9:129–134. 2013.
Article10. Chassoux F, Rodrigo S, Mellerio C, Landré E, Miquel C, Turak B, et al. Dysembryoplastic neuroepithelial tumors: an MRI-based scheme for epilepsy surgery. Neurology. 79:1699–1707. 2012.
Article11. Chong S, Phi JH, Lee JY, Kim SK. Surgical treatment of lesional mesial temporal lobe epilepsy. J Epilepsy Res. 8:6–11. 2018.
Article12. Clusmann H, Schramm J, Kral T, Helmstaedter C, Ostertun B, Fimmers R, et al. Prognostic factors and outcome after different types of resection for temporal lobe epilepsy. J Neurosurg. 97:1131–1141. 2002.
Article13. Compton JJ, Laack NN, Eckel LJ, Schomas DA, Giannini C, Meyer FB. Long-term outcomes for low-grade intracranial ganglioglioma: 30-year experience from the Mayo Clinic. J Neurosurg. 117:825–830. 2012.
Article14. Daghistani R, Miller E, Kulkarni AV, Widjaja E. Atypical characteristics and behavior of dysembryoplastic neuroepithelial tumors. Neuroradiology. 55:217–224. 2013.
Article15. Daumas-Duport C. Dysembryoplastic neuroepithelial tumours. Brain Pathol. 3:283–295. 1993.
Article16. Daumas-Duport C, Scheithauer BW, Chodkiewicz JP, Laws ER Jr, Vedrenne C. Dysembryoplastic neuroepithelial tumor: a surgically curable tumor of young patients with intractable partial seizures. Report of thirty-nine cases. Neurosurgery. 23:545–556. 1988.
Article17. Daumas-Duport C, Varlet P, Bacha S, Beuvon F, Cervera-Pierot P, Chodkiewicz JP. Dysembryoplastic neuroepithelial tumors: nonspecific histological forms -- a study of 40 cases. J Neurooncol. 41:267–280. 1999.18. Dougherty MJ, Santi M, Brose MS, Ma C, Resnick AC, Sievert AJ, et al. Activating mutations in BRAF characterize a spectrum of pediatric lowgrade gliomas. Neuro Oncol. 12:621–630. 2010.
Article19. Dudley RW, Torok MR, Gallegos DR, Mulcahy-Levy JM, Hoffman LM, Liu AK, et al. Pediatric low-grade ganglioglioma: epidemiology, treatments, and outcome analysis on 348 children from the surveillance, epidemiology, and end results database. Neurosurgery. 76:313–319. discussion 319; quiz 319-320. 2015.
Article20. Englot DJ, Berger MS, Barbaro NM, Chang EF. Factors associated with seizure freedom in the surgical resection of glioneuronal tumors. Epilepsia. 53:51–57. 2012.
Article21. Feindel W. Osler vindicated: glioma of the leg center with Jacksonian epilepsy; removal and cure, with a 50-year follow-up. Historical vignette. J Neurosurg. 111:293–300. 2009.
Article22. Goel NJ, Abdullah KG, Lang SS. Outcomes and prognostic factors in pediatric oligodendroglioma: a population-based study. Pediatr Neurosurg. 53:24–35. 2018.
Article23. Harvey AS, Jayakar P, Duchowny M, Resnick T, Prats A, Altman N, et al. Hemifacial seizures and cerebellar ganglioglioma: an epilepsy syndrome of infancy with seizures of cerebellar origin. Ann Neurol. 40:91–98. 1996.
Article24. Holthausen H, Pieper T, Kudernatsch M. Towards early diagnosis and treatment to save children from catastrophic epilepsy -- focus on epilepsy surgery. Brain Dev. 35:730–741. 2013.
Article25. Kaley T, Touat M, Subbiah V, Hollebecque A, Rodon J, Lockhart AC, et al. BRAF inhibition in BRAFV600-mutant gliomas: results from the VEBASKET study. J Clin Oncol. 36:3477–3484. 2018.
Article26. Kim NR, Wang KC, Bang JS, Choe G, Park Y, Kim SK, et al. Glioblastomatous transformation of ganglioglioma: case report with reference to molecular genetic and flow cytometric analysis. Pathol Int. 53:874–882. 2003.
Article27. Koelsche C, Wöhrer A, Jeibmann A, Schittenhelm J, Schindler G, Preusser M, et al. Mutant BRAF V600E protein in ganglioglioma is predominantly expressed by neuronal tumor cells. Acta Neuropathol. 125:891–900. 2013.
Article28. Koh HY, Kim SH, Jang J, Kim H, Han S, Lim JS, et al. BRAF somatic mutation contributes to intrinsic epileptogenicity in pediatric brain tumors. Nat Med. 24:1662–1668. 2018.
Article29. Kreiger PA, Okada Y, Simon S, Rorke LB, Louis DN, Golden JA. Losses of chromosomes 1p and 19q are rare in pediatric oligodendrogliomas. Acta Neuropathol. 109:387–392. 2005.
Article30. Lim JS, Kim WI, Kang HC, Kim SH, Park AH, Park EK, et al. Brain somatic mutations in MTOR cause focal cortical dysplasia type II leading to intractable epilepsy. Nat Med. 21:395–400. 2015.
Article31. Luyken C, Blümcke I, Fimmers R, Urbach H, Elger CE, Wiestler OD, et al. The spectrum of long-term epilepsy-associated tumors: long-term seizure and tumor outcome and neurosurgical aspects. Epilepsia. 44:822–830. 2003.
Article32. Maher CO, White JB, Scheithauer BW, Raffel C. Recurrence of dysembryoplastic neuroepithelial tumor following resection. Pediatr Neurosurg. 44:333–336. 2008.
Article33. Majores M, von Lehe M, Fassunke J, Schramm J, Becker AJ, Simon M. Tumor recurrence and malignant progression of gangliogliomas. Cancer. 113:3355–3363. 2008.
Article34. Marucci G, de Biase D, Visani M, Giulioni M, Martinoni M, Volpi L, et al. Mutant BRAF in low-grade epilepsy-associated tumors and focal cortical dysplasia. Ann Clin Transl Neurol. 1:130–134. 2014.
Article35. Ni HC, Chen SY, Chen L, Lu DH, Fu YJ, Piao YS. Angiocentric glioma: a report of nine new cases, including four with atypical histological features. Neuropathol Appl Neurobiol. 41:333–346. 2015.
Article36. Pallud J, Audureau E, Blonski M, Sanai N, Bauchet L, Fontaine D, et al. Epileptic seizures in diffuse low-grade gliomas in adults. Brain. 137(Pt 2):449–462. 2014.
Article37. Pallud J, McKhann GM. Diffuse low-grade glioma-related epilepsy. Neurosurg Clin N Am. 30:43–54. 2019.
Article38. Pekmezci M, Villanueva-Meyer JE, Goode B, Van Ziffle J, Onodera C, Grenert JP, et al. The genetic landscape of ganglioglioma. Acta Neuropathol Commun. 6:47. 2018.
Article39. Perkins SM, Mitra N, Fei W, Shinohara ET. Patterns of care and outcomes of patients with pleomorphic xanthoastrocytoma: a SEER analysis. J Neurooncol. 110:99–104. 2012.
Article40. Phi JH, Kim SK, Cho BK, Lee SY, Park SY, Park SJ, et al. Long-term surgical outcomes of temporal lobe epilepsy associated with low-grade brain tumors. Cancer. 115:5771–5779. 2009.
Article41. Phi JH, Paeng JC, Lee HS, Wang KC, Cho BK, Lee JY, et al. Evaluation of focal cortical dysplasia and mixed neuronal and glial tumors in pediatric epilepsy patients using 18F-FDG and 11C-methionine pet. J Nucl Med. 51:728–734. 2010.
Article42. Prayson RA, Fong J, Najm I. Coexistent pathology in chronic epilepsy patients with neoplasms. Mod Pathol. 23:1097–1103. 2010.
Article43. Prayson RA, Napekoski KM. Composite ganglioglioma/dysembryoplastic neuroepithelial tumor: a clinicopathologic study of 8 cases. Hum Pathol. 43:1113–1118. 2012.
Article44. Qaddoumi I, Orisme W, Wen J, Santiago T, Gupta K, Dalton JD, et al. Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol. 131:833–845. 2016.
Article45. Raghavan R, Balani J, Perry A, Margraf L, Vono MB, Cai DX, et al. Pediatric oligodendrogliomas: a study of molecular alterations on 1p and 19q using fluorescence in situ hybridization. J Neuropathol Exp Neurol. 62:530–537. 2003.
Article46. Ramkissoon LA, Horowitz PM, Craig JM, Ramkissoon SH, Rich BE, Schumacher SE, et al. Genomic analysis of diffuse pediatric low-grade gliomas identifies recurrent oncogenic truncating rearrangements in the transcription factor MYBL1. Proc Natl Acad Sci U S A. 110:8188–8193. 2013.
Article47. Rivera B, Gayden T, Carrot-Zhang J, Nadaf J, Boshari T, Faury D, et al. Germline and somatic FGFR1 abnormalities in dysembryoplastic neuroepithelial tumors. Acta Neuropathol. 131:847–863. 2016.
Article48. Rodriguez FJ, Tihan T, Lin D, McDonald W, Nigro J, Feuerstein B, et al. Clinicopathologic features of pediatric oligodendrogliomas: a series of 50 patients. Am J Surg Pathol. 38:1058–1070. 2014.49. Southwell DG, Garcia PA, Berger MS, Barbaro NM, Chang EF. Longterm seizure control outcomes after resection of gangliogliomas. Neurosurgery. 70:1406–1413. discussion 1413-1414. 2012.
Article50. Stone TJ, Keeley A, Virasami A, Harkness W, Tisdall M, Izquierdo Delgado E, et al. Comprehensive molecular characterisation of epilepsyassociated glioneuronal tumours. Acta Neuropathol. 135:115–129. 2018.
Article51. Thom M, Blümcke I, Aronica E. Long-term epilepsy-associated tumors. Brain Pathol. 22:350–379. 2012.
Article52. Tomita T, Volk JM, Shen W, Pundy T. Glioneuronal tumors of cerebral hemisphere in children: correlation of surgical resection with seizure outcomes and tumor recurrences. Childs Nerv Syst. 32:1839–1848. 2016.
Article53. You G, Sha ZY, Yan W, Zhang W, Wang YZ, Li SW, et al. Seizure characteristics and outcomes in 508 Chinese adult patients undergoing primary resection of low-grade gliomas: a clinicopathological study. Neuro Oncol. 14:230–241. 2012.
Article54. Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 45:602–612. 2013.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changing Perspectives of Epilepsy Classification with Advances in vrain Imaging Techniques
- Advances in Understanding the Molecular Biology of Brain Tumors
- Interictal EEG in Diagnosis and Assessment of Epilepsy
- Molecular Approaches for Brain Tumor Therapy;Gene Transfer and Anti-sense Oligonucleotides
- Primary Reading Epilepsy: A Case Report