Allergy Asthma Immunol Res.
2019 Sep;11(5):709-722. 10.4168/aair.2019.11.5.709.
Severe Cutaneous Adverse Reactions to Antiepileptic Drugs: A Nationwide Registry-Based Study in Korea
- Affiliations
-
- 1Department of Internal Medicine, Inje University Haeundae Paik Hospital, Busan, Korea.
- 2Drug Safety Monitoring Center, Seoul National University Hospital, Seoul, Korea.
- 3Department of Preventive Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Chungbuk National University Hospital, Cheongju, Korea.
- 5Department of Internal Medicine, Kyungpook National University Hospital, Daegu, Korea.
- 6Department of Internal Medicine, Ajou University School of Medicine, Suwon, Korea.
- 7Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 8Department of Internal Medicine, Pusan National University Hospital, Busan, Korea.
- 9Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 10Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea.
- 11Department of Internal Medicine, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, Korea.
- 12Department of Internal Medicine, Dankook University College of Medicine, Cheonan, Korea.
- 13Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea.
- 14Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Korea.
- 15Department of Internal Medicine, Inha University School of Medicine, Seoul, Korea.
- 16Department of Internal Medicine, Inje University Pusan Paik Hospital, Busan, Korea.
- 17Department of Internal Medicine, Hallym University Sacred Heart Hospital, Anyang, Korea.
- 18Department of Internal Medicine, Jeju National University Hospital, Jeju, Korea.
- 19Department of Internal Medicine, Dongguk University Ilsan Hospital, Goyang, Korea.
- 20Department of Internal Medicine, Keimyung University Dongsan Medical Center, Daegu, Korea.
- 21Department of Pharmacology, The Catholic University of Korea, College of Medicine, Seoul, Korea.
- 22Department of Internal Medicine, Ewha Womans University Hospital, Seoul, Korea.
- 23Department of Internal Medicine, Chonbuk National University Hospital, Jeonju, Korea.
- 24Department of Internal Medicine, Chonnam National University Hospital, Gwangju, Korea.
- 25Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea.
- 26Department of Internal Medicine Chosun University Hospital, Gwangju, Korea.
- 27Department of Internal medicine, Medical School of Yeungnam University, Daegu, Korea.
- 28Department of Internal Medicine, Kosin University College of Medicine, Busan Korea.
- 29Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 30Institute of Allergy and Clinical Immunology, Seoul National University Medical Research Center, Seoul, Korea.
- 31Department of Pulmonology and Allergy, Hallym University Dongtan Sacred Heart Hospital, Hallym University College of Medicine, Hwaseong, Korea. mdqueen@hallym.or.kr
- 32Allergy and Clinical Immunology Research Center, Hallym University College of Medicine, Chuncheon, Korea.
- KMID: 2452760
- DOI: http://doi.org/10.4168/aair.2019.11.5.709
Abstract
- PURPOSE
Severe cutaneous adverse reactions (SCARs), including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) to antiepileptic drug (AED), are rare, but result in significant morbidity and mortality. We investigated the major culprit drugs, clinical characteristics, and clinical course and outcomes of AED-induced SCARs using a nationwide registry in Korea.
METHODS
A total of 161 patients with AED-induced SCARs from 28 referral hospitals were analyzed. The causative AEDs, clinical characteristics, organ involvements, details of treatment, and outcomes were evaluated. We compared the clinical and laboratory parameters between SJS/TEN and DRESS according to the leading causative drugs. We further determined risk factors for prolonged hospitalization in AED-induced SCARs.
RESULTS
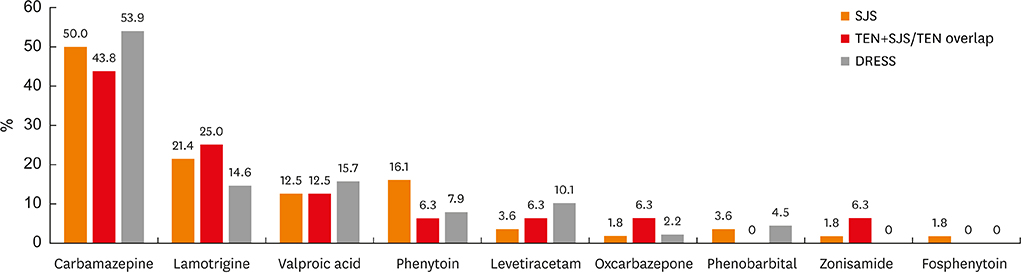
Carbamazepine and lamotrigine were the most common culprit drugs causing SCARs. Valproic acid and levetiracetam also emerged as the major causative agents. The disease duration and hospital stay in carbamazepine-induced SJS/TEN were shorter than those in other AEDs (P< 0.05, respectively). In younger patients, lamotrigine caused higher incidences of DRESS than other drugs (P= 0.045). Carbamazepine, the most common culprit drug for SCARs, was associated with a favorable outcome related with prolonged hospitalization in SJS (odds ratio, 0.12; 95% confidence interval, 0.02-0.63, P= 0.12), and thrombocytopenia was found to be a risk factor for prolonged hospitalization in DRESS.
CONCLUSION
This was the first large-scale epidemiological study of AED-induced SCARs in Korea. Valproic acid and levetiracetam were the significant emerging AEDs causing SCARs in addition to the well-known offending AEDs such as carbamazepine and lamotrigine. Carbamazepine was associated with reduced hospitalization, but thrombocytopenia was a risk factor for prolonged hospitalization. Our results suggest that the clinical characteristics and clinical courses of AED-induced SCARs might vary according to the individual AEDs.
MeSH Terms
Figure
Cited by 1 articles
-
Adverse Skin Reactions with Antiepileptic Drugs Using Korea Adverse Event Reporting System Database, 2008–2017
Hyun Kyung Kim, Dae Yeon Kim, Eun-Kee Bae, Dong Wook Kim
J Korean Med Sci. 2020;35(4):. doi: 10.3346/jkms.2020.35.e17.
Reference
-
1. Ye YM, Thong BY, Park HS. Hypersensitivity to antiepileptic drugs. Immunol Allergy Clin North Am. 2014; 34:633–643.
Article2. Błaszczyk B, Szpringer M, Czuczwar SJ, Lasoń W. Single centre 20 year survey of antiepileptic drug-induced hypersensitivity reactions. Pharmacol Rep. 2013; 65:399–409.
Article3. Zaccara G, Franciotta D, Perucca E. Idiosyncratic adverse reactions to antiepileptic drugs. Epilepsia. 2007; 48:1223–1244.
Article4. Perucca P, Gilliam FG. Adverse effects of antiepileptic drugs. Lancet Neurol. 2012; 11:792–802.
Article5. Gilliam F, Carter J, Vahle V. Tolerability of antiseizure medications: implications for health outcomes. Neurology. 2004; 63:S9–12.
Article6. Yang CY, Dao RL, Lee TJ, Lu CW, Yang CH, Hung SI, et al. Severe cutaneous adverse reactions to antiepileptic drugs in Asians. Neurology. 2011; 77:2025–2033.
Article7. Ordoñez L, Salgueiro E, Jimeno FJ, Manso G. Spontaneous reporting of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with antiepileptic drugs. Eur Rev Med Pharmacol Sci. 2015; 19:2732–2737.8. Arif H, Buchsbaum R, Weintraub D, Koyfman S, Salas-Humara C, Bazil CW, et al. Comparison and predictors of rash associated with 15 antiepileptic drugs. Neurology. 2007; 68:1701–1709.
Article9. Wang XQ, Lv B, Wang HF, Zhang X, Yu SY, Huang XS, et al. Lamotrigine induced DIHS/DRESS: manifestations, treatment, and outcome in 57 patients. Clin Neurol Neurosurg. 2015; 138:1–7.
Article10. Mockenhaupt M, Messenheimer J, Tennis P, Schlingmann J. Risk of Stevens-Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptics. Neurology. 2005; 64:1134–1138.
Article11. Schlienger RG, Oh PI, Knowles SR, Shear NH. Quantifying the costs of serious adverse drug reactions to antiepileptic drugs. Epilepsia. 1998; 39:Suppl 7. S27–32.
Article12. Suh DC, Woodall BS, Shin SK, Hermes-De Santis ER. Clinical and economic impact of adverse drug reactions in hospitalized patients. Ann Pharmacother. 2000; 34:1373–1379.
Article13. Bessmertny O, Pham T. Antiepileptic hypersensitivity syndrome: clinicians beware and be aware. Curr Allergy Asthma Rep. 2002; 2:34–39.
Article14. Sun D, Shi YW, Liu ZS, Liao WP, Wang FL, Wu GF, et al. Hypersensitivity syndrome reactions to antiepileptic drugs, clinical characteristic and association with HLA-B*1502. Zhonghua Yi Xue Za Zhi. 2010; 90:2763–2766.15. Park HW, Kim SH, Chang YS, Kim SH, Jee YK, Lee AY, et al. The Fas signaling pathway is a common genetic risk factor for severe cutaneous drug adverse reactions across diverse drugs. Allergy Asthma Immunol Res. 2018; 10:555–561.
Article16. Kang DY, Ahn KM, Kang HR, Cho SH. Past, present, and future of pharmacovigilance in Korea. Asia Pac Allergy. 2017; 7:173–178.
Article17. Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993; 129:92–96.
Article18. Kardaun SH, Sidoroff A, Valeyrie-Allanore L, Halevy S, Davidovici BB, Mockenhaupt M, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007; 156:609–611.
Article19. Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: a clinician's guide to terminology, documentation, and reporting. Ann Intern Med. 2004; 140:795–801.
Article20. Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000; 356:1255–1259.
Article21. Kang D, Kang M, Kim S, Kim S, Jin H, Ye Y, et al. Surveillance of severe cutaneous adverse reactions in Korea based on a nationwide registry [abstract]. Allergy. 2017; 72:112.22. Yang MS, Kang MG, Jung JW, Song WJ, Kang HR, Cho SH, et al. Clinical features and prognostic factors in severe cutaneous drug reactions. Int Arch Allergy Immunol. 2013; 162:346–354.
Article23. Hamer HM, Morris HH. Hypersensitivity syndrome to antiepileptic drugs: a review including new anticonvulsants. Cleve Clin J Med. 1999; 66:239–245.24. Gogtay NJ, Bavdekar SB, Kshirsagar NA. Anticonvulsant hypersensitivity syndrome: a review. Expert Opin Drug Saf. 2005; 4:571–581.
Article25. Wolf R, Matz H, Marcos B, Orion E. Drug rash with eosinophilia and systemic symptoms vs toxic epidermal necrolysis: the dilemma of classification. Clin Dermatol. 2005; 23:311–314.
Article26. Teraki Y, Shibuya M, Izaki S. Stevens-Johnson syndrome and toxic epidermal necrolysis due to anticonvulsants share certain clinical and laboratory features with drug-induced hypersensitivity syndrome, despite differences in cutaneous presentations. Clin Exp Dermatol. 2010; 35:723–728.
Article27. Kim JY, Lee J, Ko YJ, Shin JY, Jung SY, Choi NK, et al. Multi-indication carbamazepine and the risk of severe cutaneous adverse drug reactions in Korean elderly patients: a Korean health insurance data-based study. PLoS One. 2013; 8:e83849.
Article28. Galindo PA, Borja J, Gómez E, Mur P, Gudín M, García R, et al. Anticonvulsant drug hypersensitivity. J Investig Allergol Clin Immunol. 2002; 12:299–304.29. Wang XQ, Shi XB, Au R, Chen FS, Wang F, Lang SY. Influence of chemical structure on skin reactions induced by antiepileptic drugs--the role of the aromatic ring. Epilepsy Res. 2011; 94:213–217.
Article30. Dar WR, Sofi N, Latief M, Dar IA, Kasana BA. Levetiracetam induced drug reaction with eosinophilia and systemic symptom syndrome. Indian J Dermatol. 2016; 61:235.
Article31. Messenheimer J, Mullens EL, Giorgi L, Young F. Safety review of adult clinical trial experience with lamotrigine. Drug Saf. 1998; 18:281–296.
Article32. Krakow K, Walker M, Otoul C, Sander JW. Long-term continuation of levetiracetam in patients with refractory epilepsy. Neurology. 2001; 56:1772–1774.
Article33. Mahar PD, Wasiak J, Hii B, Cleland H, Watters DA, Gin D, et al. A systematic review of the management and outcome of toxic epidermal necrolysis treated in burns centres. Burns. 2014; 40:1245–1254.
Article34. Sekula P, Dunant A, Mockenhaupt M, Naldi L, Bouwes Bavinck JN, Halevy S, et al. Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Invest Dermatol. 2013; 133:1197–1204.
Article35. Hsu DY, Brieva J, Silverberg NB, Silverberg JI. Morbidity and mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis in United States adults. J Invest Dermatol. 2016; 136:1387–1397.
Article36. Yang MS, Lee JY, Kim J, Kim GW, Kim BK, Kim JY, et al. Incidence of Stevens-Johnson syndrome and toxic epidermal necrolysis: a nationwide population-based study using national health insurance database in Korea. PLoS One. 2016; 11:e0165933.
Article37. Chan L, Cook DK. A 10-year retrospective cohort study of the management of toxic epidermal necrolysis and Stevens-Johnson syndrome in a New South Wales state referral hospital from 2006 to 2016. Int J Dermatol. 2019.
Article38. Lee HY, Dunant A, Sekula P, Mockenhaupt M, Wolkenstein P, Valeyrie-Allanore L, et al. The role of prior corticosteroid use on the clinical course of Stevens-Johnson syndrome and toxic epidermal necrolysis: a case-control analysis of patients selected from the multinational EuroSCAR and RegiSCAR studies. Br J Dermatol. 2012; 167:555–562.
Article39. Zimmermann S, Sekula P, Venhoff M, Motschall E, Knaus J, Schumacher M, et al. Systemic immunomodulating therapies for Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2017; 153:514–522.40. Valeyrie-Allanore L, Bastuji-Garin S, Guégan S, Ortonne N, Bagot M, Roujeau JC, et al. Prognostic value of histologic features of toxic epidermal necrolysis. J Am Acad Dermatol. 2013; 68:e29–35.
Article41. Schulz JT, Sheridan RL, Ryan CM, MacKool B, Tompkins RG. A 10-year experience with toxic epidermal necrolysis. J Burn Care Rehabil. 2000; 21:199–204.
Article42. Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000; 115:149–153.43. Wei CH, Chung-Yee Hui R, Chang CJ, Ho HC, Yang CH, Lin YJ, et al. Identifying prognostic factors for drug rash with eosinophilia and systemic symptoms (DRESS). Eur J Dermatol. 2011; 21:930–937.
Article44. Walsh SA, Creamer D. Drug reaction with eosinophilia and systemic symptoms (DRESS): a clinical update and review of current thinking. Clin Exp Dermatol. 2011; 36:6–11.
Article45. Chiou CC, Yang LC, Hung SI, Chang YC, Kuo TT, Ho HC, et al. Clinicopathological features and prognosis of drug rash with eosinophilia and systemic symptoms: a study of 30 cases in Taiwan. J Eur Acad Dermatol Venereol. 2008; 22:1044–1049.46. Kardaun SH, Sekula P, Valeyrie-Allanore L, Liss Y, Chu CY, Creamer D, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013; 169:1071–1080.
Article47. Kano Y, Ishida T, Hirahara K, Shiohara T. Visceral involvements and long-term sequelae in drug-induced hypersensitivity syndrome. Med Clin North Am. 2010; 94:743–759.
Article48. Chung WH, Hung SI, Chen YT. Genetic predisposition of life-threatening antiepileptic-induced skin reactions. Expert Opin Drug Saf. 2010; 9:15–21.
Article49. Mullan KA, Anderson A, Illing PT, Kwan P, Purcell AW, Mifsud NA. HLA-associated antiepileptic drug-induced cutaneous adverse reactions. HLA. 2019; 93:417–435.
Article