Dement Neurocogn Disord.
2019 Jun;18(2):33-46. 10.12779/dnd.2019.18.2.33.
A Therapeutic Strategy for Alzheimer's Disease Focused on Immune-inflammatory Modulation
- Affiliations
-
- 1Department of Neurology, Hanyang University College of Medicine, Seoul, Korea. kimsh1@hanyang.ac.kr
- 2Department of Neurology, Konkuk University School of Medicine, Seoul, Korea.
- 3Department of Laboratory Medicine, Hanyang University College of Medicine, Seoul, Korea.
- 4Stem Cell Neuroplasticity Research Group, Kyungpook National University, Daegu, Korea.
- 5Department of Physiology, Cell and Matrix Research Institute, School of Medicine, Kyungpook National University, Daegu, Korea.
- 6Department of Laboratory Animal Medicine, College of Veterinary Medicine, Kyungpook National University, Daegu, Korea.
- KMID: 2451000
- DOI: http://doi.org/10.12779/dnd.2019.18.2.33
Abstract
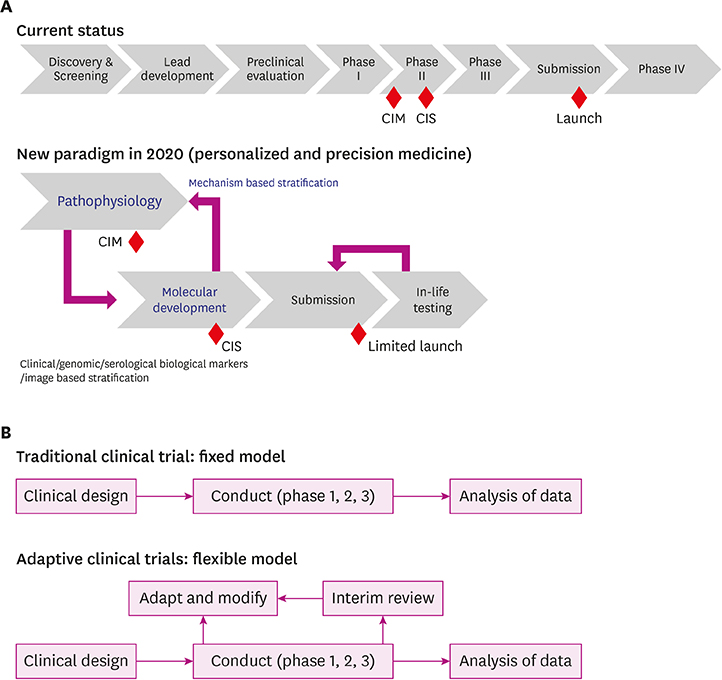
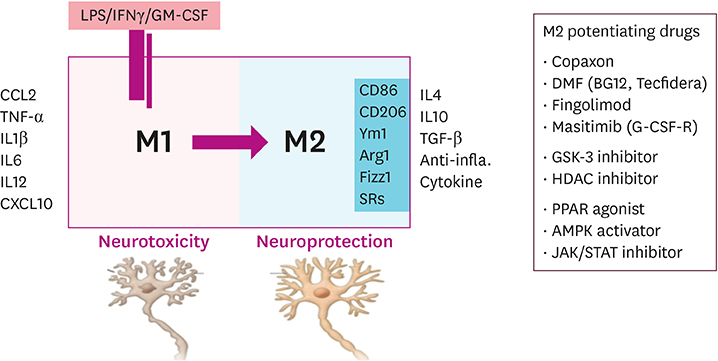
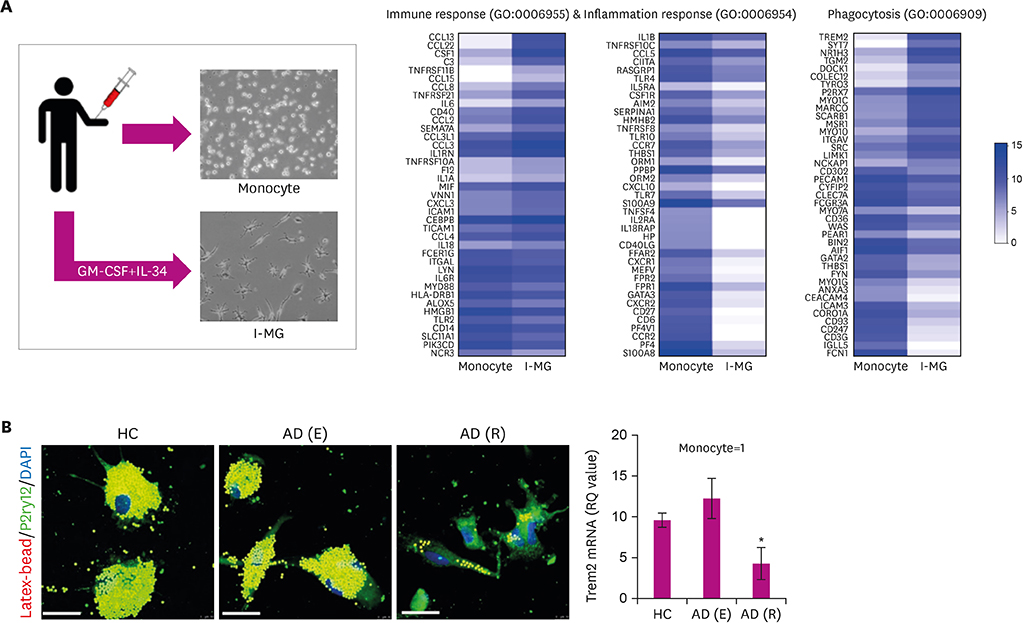
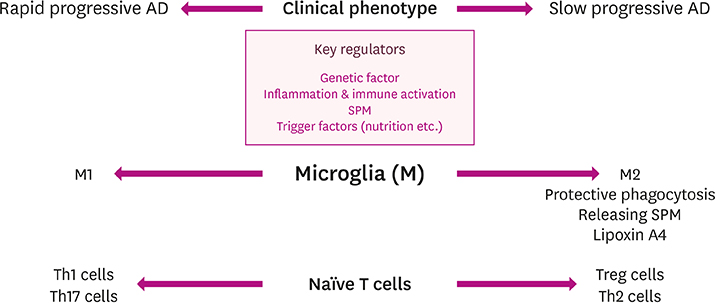
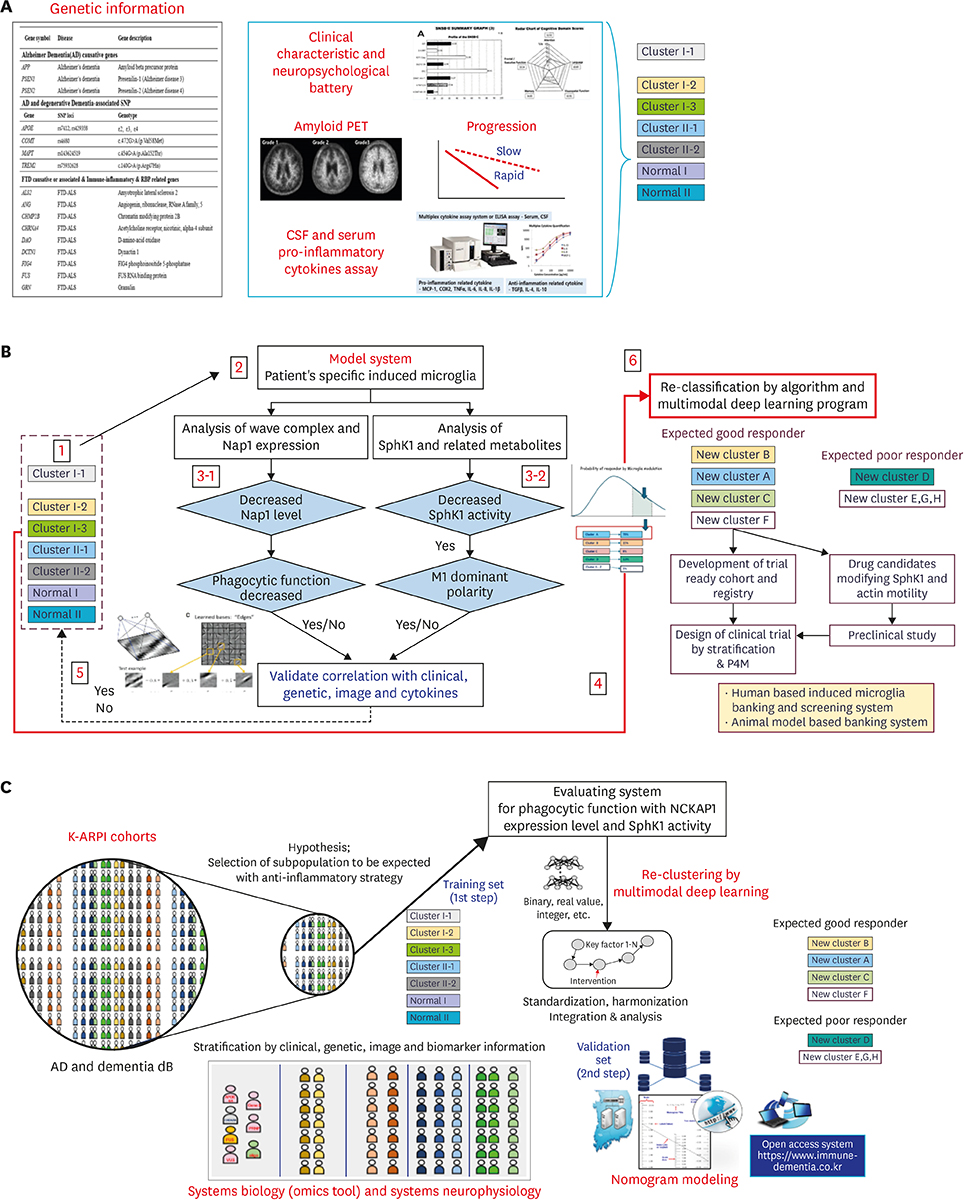
- Alzheimer's disease (AD), the most common form of dementia, has emerged as a major global public health challenge. However, the complexity of AD in its biological, genetic, and clinical aspects has hindered the development of effective therapeutic agents. Research plans that integrate new drug discoveries are urgently needed, including those based on novel and reliable biomarkers that reflect not only clinical phenotype, but also genetic and neuroimaging information. Therapeutic strategies such as stratification (i.e., subgrouping of patients having similar clinical characteristics or genetic background) and personalized medicine could be set as new directions for developing effective drugs for AD. In this review, we describe a therapeutic strategy that is based on immune-inflammation modulation for a subgroup of AD and related dementias, arguing that the use of stratification and personalized medicine is a promising way to achieve targeted medicine. The Korean AD Research Platform Initiative based on Immune-Inflammatory biomarkers (K-ARPI) has recently launched a strategy to develop novel biomarkers to identify a subpopulation of patients with AD and to develop new drug candidates for delaying the progression of AD by modulating toxic immune inflammatory response. Sphingosine kinase 1 (SphK1) and its metabolites, triggering receptor expressed on myeloid cells-2 (TREM2) related signals, and actin motility related proteins including Nck-associated protein 1 (Nap1) were selected as promising targets to modulate neuroinflammation. Their roles in stratification and personalized medicine will be discussed.
MeSH Terms
Figure
Reference
-
1. Alzheimer's Association. 2017 Alzheimer's disease facts and figures. Alzheimers Dement. 2017; 13:325–373.2. World Health Organization. Global Action Plan on the Public Health Response to Dementia 2017–2025. Geneva: World Health Organization;2017.3. Prince M, Guerchet M, Prina M. The Epidemiology and Impact of Dementia: Current State and Future Trends. Geneva: World Health Organization;2015. p. –. .4. National Institute of Dementia. Korean Dementia Observatory 2016. Seongnam: National Institute of Dementia;2017.5. Karantzoulis S, Galvin JE. Distinguishing Alzheimer's disease from other major forms of dementia. Expert Rev Neurother. 2011; 11:1579–1591.
Article6. McManus RM, Heneka MT. Role of neuroinflammation in neurodegeneration: new insights. Alzheimers Res Ther. 2017; 9:14.
Article7. Mayeux R, Stern Y, Spanton S. Heterogeneity in dementia of the Alzheimer type: evidence of subgroups. Neurology. 1985; 35:453–461.
Article8. Yiannopoulou KG, Papageorgiou SG. Current and future treatments for Alzheimer's disease. Ther Adv Neurol Disorder. 2013; 6:19–33.
Article9. Zhao X, Zheng X, Fan TP, Li Z, Zhang Y, Zheng J. A novel drug discovery strategy inspired by traditional medicine philosophies. Science. 2015; 347:S38–S40.10. Herrup K, Carrillo MC, Schenk D, Cacace A, Desanti S, Fremeau R, et al. Beyond amyloid: getting real about nonamyloid targets in Alzheimer's disease. Alzheimers Dement. 2013; 9:452–458.e1.
Article11. Hampel H, Vergallo A, Giorgi FS, Kim SH, Depypere H, Graziani M, et al. Precision medicine and drug development in Alzheimer's disease: the importance of sexual dimorphism and patient stratification. Front Neuroendocrinol. 2018; 50:31–51.
Article12. Hampel H, Toschi N, Babiloni C, Baldacci F, Black KL, Bokde AL, et al. Revolution of Alzheimer precision neurology. Passageway of systems biology and neurophysiology. J Alzheimers Dis. 2018; 64:S47–S105.13. Cummings JL, Morstorf T, Zhong LK. Alzheimer's disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther. 2014; 6:37.
Article14. Morgan P, Brown DG, Lennard S, Anderton MJ, Barrett JC, Eriksson U, et al. Impact of a five-dimensional framework on R&D productivity at AstraZeneca. Nat Rev Drug Discov. 2018; 17:167–181.
Article15. Finger E, Berry S, Cummings J, Coleman K, Hsiung R, Feldman HH, et al. Adaptive crossover designs for assessment of symptomatic treatments targeting behaviour in neurodegenerative disease: a phase 2 clinical trial of intranasal oxytocin for frontotemporal dementia (FOXY). Alzheimers Res Ther. 2018; 10:102.
Article16. Lam B, Masellis M, Freedman M, Stuss DT, Black SE. Clinical, imaging, and pathological heterogeneity of the Alzheimer's disease syndrome. Alzheimers Res Ther. 2013; 5:1.
Article17. Schmidt C, Wolff M, Weitz M, Bartlau T, Korth C, Zerr I. Rapidly progressive Alzheimer disease. Arch Neurol. 2011; 68:1124–1130.
Article18. Pricewaterhouse Coopers. Pharma 2020: the vision. Which path will you take? [Internet]. London: Pricewaterhouse Coopers;2007. cited 2007 June. Available from: https://www.pwc.com/gx/en/pharma-life-sciences/pdf/pharma2020final.pdf.19. Pallmann P, Bedding AW, Choodari-Oskooei B, Dimairo M, Flight L, Hampson LV, et al. Adaptive designs in clinical trials: why use them, and how to run and report them. BMC Med. 2018; 16:29.
Article20. Heneka MT, Kummer MP, Latz E. Innate immune activation in neurodegenerative disease. Nat Rev Immunol. 2014; 14:463–477.
Article21. Hooten KG, Beers DR, Zhao W, Appel SH. Protective and toxic neuroinflammation in amyotrophic lateral sclerosis. Neurotherapeutics. 2015; 12:364–375.
Article22. Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, et al. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015; 14:388–405.
Article23. Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011; 91:461–553.
Article24. Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013; 38:792–804.
Article25. Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo . Science. 2005; 308:1314–1318.
Article26. Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, et al. A unique microglia type associated with restricting development of Alzheimer's disease. Cell. 2017; 169:1276–1290.e17.
Article27. Deczkowska A, Keren-Shaul H, Weiner A, Colonna M, Schwartz M, Amit I. Disease-associated microglia: a universal immune sensor of neurodegeneration. Cell. 2018; 173:1073–1081.
Article28. Michell-Robinson MA, Touil H, Healy LM, Owen DR, Durafourt BA, Bar-Or A, et al. Roles of microglia in brain development, tissue maintenance and repair. Brain. 2015; 138:1138–1159.
Article29. Andreasson KI, Bachstetter AD, Colonna M, Ginhoux F, Holmes C, Lamb B, et al. Targeting innate immunity for neurodegenerative disorders of the central nervous system. J Neurochem. 2016; 138:653–693.
Article30. Song GJ, Suk K. Pharmacological modulation of functional phenotypes of microglia in neurodegenerative diseases. Front Aging Neurosci. 2017; 9:139.
Article31. Malik M, Parikh I, Vasquez JB, Smith C, Tai L, Bu G, et al. Genetics ignite focus on microglial inflammation in Alzheimer's disease. Mol Neurodegener. 2015; 10:52.
Article32. Noh MY, Lim SM, Oh KW, Cho KA, Park J, Kim KS, et al. Mesenchymal stem cells modulate the functional properties of microglia via TGF-β secretion. Stem Cells Transl Med. 2016; 5:1538–1549.
Article33. Bianchin MM, Capella HM, Chaves DL, Steindel M, Grisard EC, Ganev GG, et al. Nasu-Hakola disease (polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy--PLOSL): a dementia associated with bone cystic lesions. From clinical to genetic and molecular aspects. Cell Mol Neurobiol. 2004; 24:1–24.
Article34. Hickman SE, El Khoury J. TREM2 and the neuroimmunology of Alzheimer's disease. Biochem Pharmacol. 2014; 88:495–498.
Article35. Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer's disease model. Cell. 2015; 160:1061–1071.
Article36. Slattery CF, Beck JA, Harper L, Adamson G, Abdi Z, Uphill J, et al. R47H TREM2 variant increases risk of typical early-onset Alzheimer's disease but not of prion or frontotemporal dementia. Alzheimers Dement. 2014; 10:602–608.e4.
Article37. Cady J, Koval ED, Benitez BA, Zaidman C, Jockel-Balsarotti J, Allred P, et al. TREM2 variant p.R47H as a risk factor for sporadic amyotrophic lateral sclerosis. JAMA Neurol. 2014; 71:449–453.
Article38. Palmqvist S, Mattsson N, Hansson O. Alzheimer's Disease Neuroimaging Initiative. Cerebrospinal fluid analysis detects cerebral amyloid-β accumulation earlier than positron emission tomography. Brain. 2016; 139:1226–1236.
Article39. Suárez-Calvet M, Kleinberger G, Araque Caballero MÁ, Brendel M, Rominger A, Alcolea D, et al. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer's disease and associate with neuronal injury markers. EMBO Mol Med. 2016; 8:466–476.
Article40. David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011; 12:388–399.
Article41. Desikan RS, Fan CC, Wang Y, Schork AJ, Cabral HJ, Cupples LA, et al. Genetic assessment of age-associated Alzheimer disease risk: development and validation of a polygenic hazard score. PLoS Med. 2017; 14:e1002258.
Article42. Seshadri S, Fitzpatrick AL, Ikram MA, DeStefano AL, Gudnason V, Boada M, et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010; 303:1832–1840.
Article43. Boillée S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006; 312:1389–1392.
Article44. Zhao W, Beers DR, Hooten KG, Sieglaff DH, Zhang A, Kalyana-Sundaram S, et al. Characterization of gene expression phenotype in ALS monocytes. JAMA Neurol. 2017; 74:677–685.
Article45. Frakes AE, Ferraiuolo L, Haidet-Phillips AM, Schmelzer L, Braun L, Miranda CJ, et al. Microglia induce motor neuron death via the classical NF-κB pathway in amyotrophic lateral sclerosis. Neuron. 2014; 81:1009–1023.
Article46. Turner MR, Cagnin A, Turkheimer FE, Miller CC, Shaw CE, Brooks DJ, et al. Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C](R)-PK11195 positron emission tomography study. Neurobiol Dis. 2004; 15:601–609.
Article47. Beers DR, Henkel JS, Xiao Q, Zhao W, Wang J, Yen AA, et al. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2006; 103:16021–16026.
Article48. Komine O, Yamanaka K. Neuroinflammation in motor neuron disease. Nagoya J Med Sci. 2015; 77:537–549.49. Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011; 11:403–415.
Article50. Nayak D, Huo Y, Kwang WX, Pushparaj PN, Kumar SD, Ling EA, et al. Sphingosine kinase 1 regulates the expression of proinflammatory cytokines and nitric oxide in activated microglia. Neuroscience. 2010; 166:132–144.
Article51. Lee JY, Han SH, Park MH, Baek B, Song IS, Choi MK, et al. Neuronal SphK1 acetylates COX2 and contributes to pathogenesis in a model of Alzheimer's disease. Nat Commun. 2018; 9:1479–1497.
Article52. Pchejetski D, Nunes J, Coughlan K, Lall H, Pitson SM, Waxman J, et al. The involvement of sphingosine kinase 1 in LPS-induced Toll-like receptor 4-mediated accumulation of HIF-1α protein, activation of ASK1 and production of the pro-inflammatory cytokine IL-6. Immunol Cell Biol. 2011; 89:268–274.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Immune-Inflammatory Mechanisms in Alzheimer's Disease and Other Degenerative Diseases
- Microbial Modulation in Inflammatory Bowel Diseases
- The role of PI3K/AKT pathway and its therapeutic possibility in Alzheimer's disease
- Significance of Non-Alzheimer Dementia
- Damage-Associated Molecular Patterns in Inflammatory Diseases