Endocrinol Metab.
2019 Jun;34(2):203-212. 10.3803/EnM.2019.34.2.203.
Expression of NF2 Modulates the Progression of BRAFV600E Mutated Thyroid Cancer Cells
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. wongukim@amc.seoul.kr
- KMID: 2450521
- DOI: http://doi.org/10.3803/EnM.2019.34.2.203
Abstract
- BACKGROUND
We previously reported the frequent neurofibromatosis 2 (NF2) gene mutations in anaplastic thyroid cancers in association with the BRAF V600E mutation. We aimed to investigate the role of NF2 in thyroid cancer with BRAF mutation.
METHODS
To identify the function of NF2 in thyroid cancers, we investigated the changes in cell proliferation, colon formation, migration and invasion of thyroid cancer cells (8505C, BHT101, and KTC-1) with BRAF V600E mutation after overexpression and knock-down of NF2. We also examined how cell proliferation changed when NF2 was mutagenized. Human NF2 expression in papillary thyroid carcinoma (PTC) was analyzed using the The Cancer Genome Atlas (TCGA) data.
RESULTS
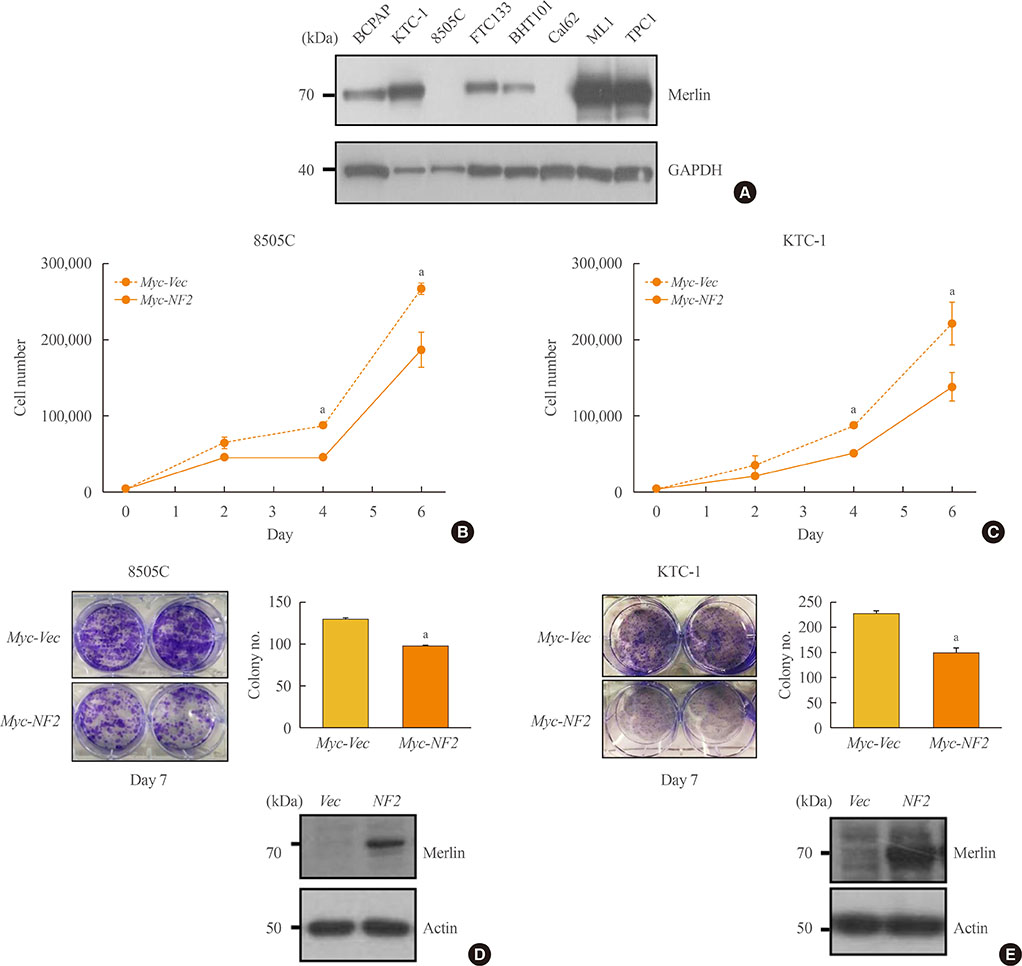
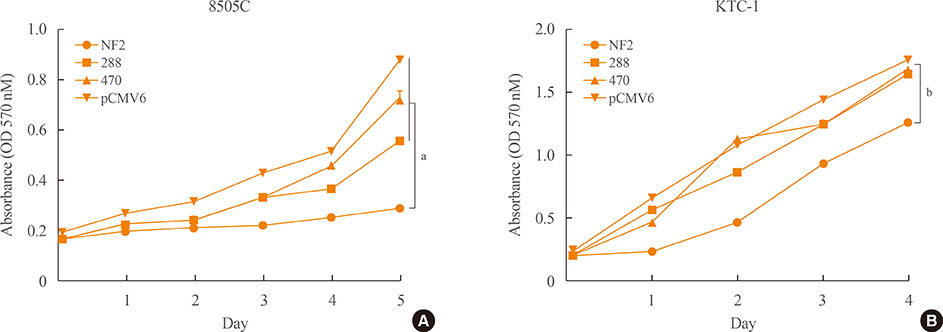
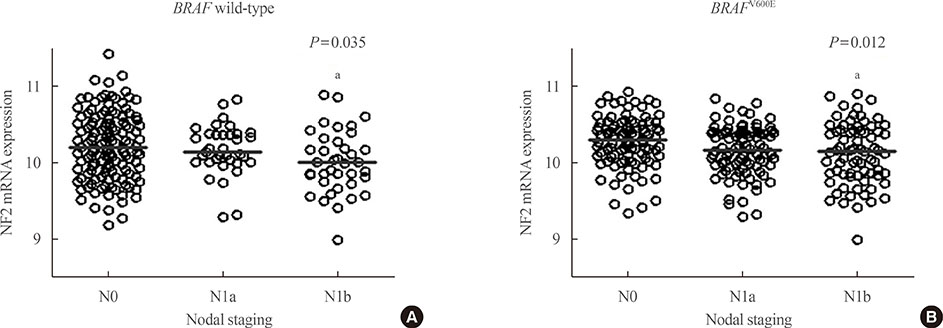
First, NF2 was overexpressed in 8505C and KTC-1 cells. Compared to control, NF2 overexpressed group of both thyroid cancer cells showed significant inhibition in cell proliferation and colony formation. These results were also confirmed by cell migration and invasion assay. After knock-down of NF2 in 8505C cells, there were no significant changes in cell proliferation and colony formation, compared with the control group. However, after mutagenized S288* and Q470* sites of NF2 gene, the cell proliferation increased compared to NF2 overexpression group. In the analysis of TCGA data, the mRNA expression of NF2 was significantly decreased in PTCs with lateral cervical lymph node (LN) metastasis compared with PTCs without LN metastasis.
CONCLUSION
Our study suggests that NF2 might play a role as a tumor suppressor in thyroid cancer with BRAF mutation. More studies are needed to elucidate the mechanism how NF2 acts in thyroid cancer with BRAF mutation.
MeSH Terms
Figure
Reference
-
1. Jung KW, Won YJ, Oh CM, Kong HJ, Lee DH, Lee KH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2014. Cancer Res Treat. 2017; 49:292–305.
Article2. Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest. 2016; 126:1052–1066.
Article3. Swaak-Kragten AT, de Wilt JH, Schmitz PI, Bontenbal M, Levendag PC. Multimodality treatment for anaplastic thyroid carcinoma: treatment outcome in 75 patients. Radiother Oncol. 2009; 92:100–104.4. Ain KB, Egorin MJ, DeSimone PA. Collaborative Anaplastic Thyroid Cancer Health Intervention Trials (CATCHIT) Group. Treatment of anaplastic thyroid carcinoma with paclitaxel: phase 2 trial using ninety-six-hour infusion. Thyroid. 2000; 10:587–594.
Article5. Jeon MJ, Chun SM, Kim D, Kwon H, Jang EK, Kim TY, et al. Genomic alterations of anaplastic thyroid carcinoma detected by targeted massive parallel sequencing in a BRAF(V600E) mutation-prevalent area. Thyroid. 2016; 26:683–690.
Article6. Gutmann DH. The neurofibromatoses: when less is more. Hum Mol Genet. 2001; 10:747–755.
Article7. James MF, Han S, Polizzano C, Plotkin SR, Manning BD, Stemmer-Rachamimov AO, et al. NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol Cell Biol. 2009; 29:4250–4261.
Article8. Lopez-Lago MA, Okada T, Murillo MM, Socci N, Giancotti FG. Loss of the tumor suppressor gene NF2, encoding merlin, constitutively activates integrin-dependent mTORC1 signaling. Mol Cell Biol. 2009; 29:4235–4249.9. Guerrero PA, Yin W, Camacho L, Marchetti D. Oncogenic role of Merlin/NF2 in glioblastoma. Oncogene. 2015; 34:2621–2630.
Article10. Petrilli AM, Fernandez-Valle C. Role of Merlin/NF2 inactivation in tumor biology. Oncogene. 2016; 35:537–548.
Article11. Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014; 94:1287–1312.
Article12. Garcia-Rendueles ME, Ricarte-Filho JC, Untch BR, Landa I, Knauf JA, Voza F, et al. NF2 loss promotes oncogenic RAS-induced thyroid cancers via YAP-dependent transactivation of RAS proteins and sensitizes them to MEK inhibition. Cancer Discov. 2015; 5:1178–1193.
Article13. Yu C, Zhang L, Luo D, Yan F, Liu J, Shao S, et al. MicroRNA-146b-3p promotes cell metastasis by directly targeting NF2 in human papillary thyroid cancer. Thyroid. 2018; 09. 22. DOI: 10.1089/thy.2017.0626. [Epub].
Article14. Jeon MJ, Lim S, You MH, Park Y, Song DE, Sim S, et al. The role of Slit2 as a tumor suppressor in thyroid cancer. Mol Cell Endocrinol. 2019; 483:87–96.
Article15. Suh S, Kim YH, Goh TS, Jeong DC, Lee CS, Jang JY, et al. mRNA expression of SLC5A5 an SLC2A family genes in papillary thyroid cancer: an analysis of The Cancer Genome Atlas. Yonsei Med J. 2018; 59:746–753.16. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–1474.
Article17. Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003; 3:362–374.
Article18. Poulikakos PI, Xiao GH, Gallagher R, Jablonski S, Jhanwar SC, Testa JR. Re-expression of the tumor suppressor NF2/merlin inhibits invasiveness in mesothelioma cells and negatively regulates FAK. Oncogene. 2006; 25:5960–5968.
Article19. Schlaepfer DD, Mitra SK. Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev. 2004; 14:92–101.
Article20. Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005; 6:56–68.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Model Development of Papillary Thyroid Carcinoma by BRAFV600E Transgenic Mice
- Expression of miRNA 146a/b, 221 and 222 in Thyroid Cancer
- Expression of Sodium-Iodide Symporter Depending on Mutational Status and Lymphocytic Thyroiditis in Papillary Thyroid Carcinoma
- Clinical and Genetic Overview of Neurofibromatosis Type 2 (NF2)
- SOX12 Promotes Thyroid Cancer Cell Proliferation and Invasion by Regulating the Expression of POU2F1 and POU3F1