Korean J Physiol Pharmacol.
2019 Jul;23(4):263-270. 10.4196/kjpp.2019.23.4.263.
Hepatoprotective effect of sodium hydrosulfide on hepatic encephalopathy in rats
- Affiliations
-
- 1Department of Pharmacology, College of Pharmacy, Chung-Ang University, Seoul 06974, Korea. udsohn@cau.ac.kr
- KMID: 2450493
- DOI: http://doi.org/10.4196/kjpp.2019.23.4.263
Abstract
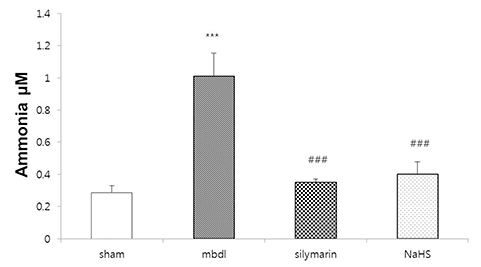
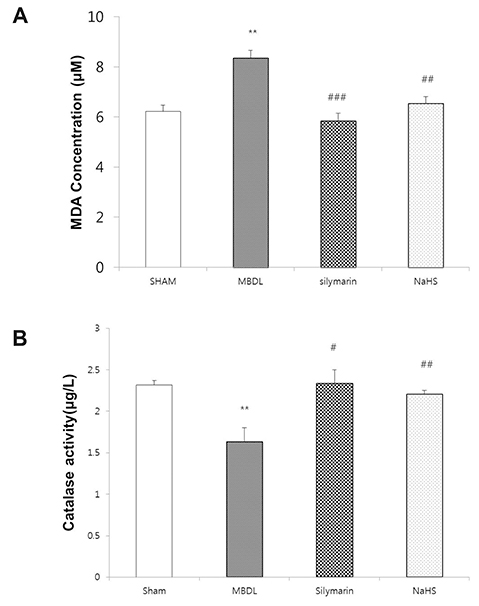
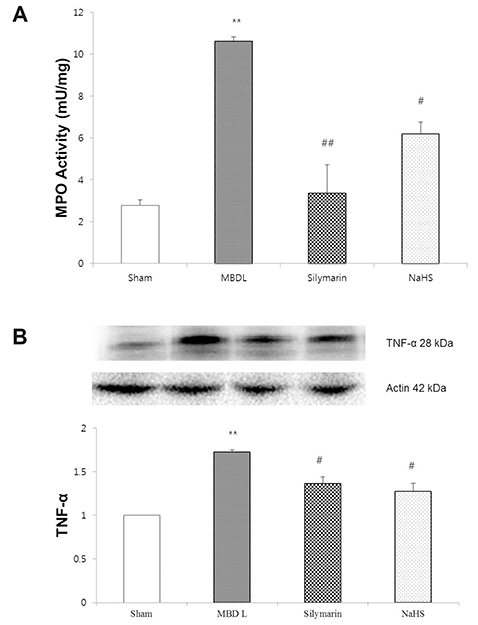
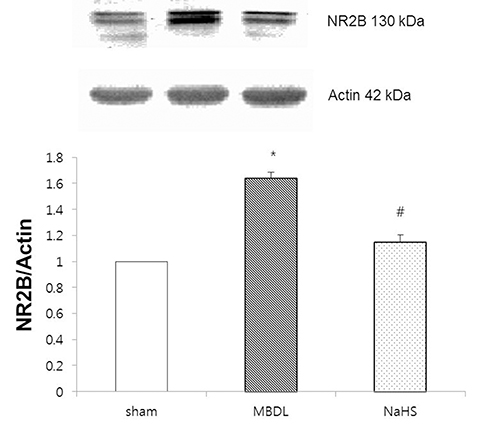
- Hydrogen sulfide is well-known to exhibit anti-inflammatory and cytoprotective activities, and also has protective effects in the liver. This study aimed to examine the protective effect of hydrogen sulfide in rats with hepatic encephalopathy, which was induced by mild bile duct ligation. In this rat model, bile ducts were mildly ligated for 26 days. Rats were treated for the final 5 days with sodium hydrosulfide (NaHS). NaHS (25 µmol/kg), 0.5% sodium carboxymethyl cellulose, or silymarin (100 mg/kg) was administered intraperitoneally once per day for 5 consecutive days. Mild bile duct ligation caused hepatotoxicity and inflammation in rats. Intraperitoneal NaHS administration reduced levels of aspartate aminotransferase and alanine aminotransferase, which are indicators of liver disease, compared to levels in the control mild bile duct ligation group. Levels of ammonia, a major causative factor of hepatic encephalopathy, were also significantly decreased. Malondialdehyde, myeloperoxidase, catalase, and tumor necrosis factor-α levels were measured to confirm antioxidative and anti-inflammatory effects. N-Methyl-D-aspartic acid (NMDA) receptors with neurotoxic activity were assessed for subunit NMDA receptor subtype 2B. Based on these data, NaHS is suggested to exhibit hepatoprotective effects and guard against neurotoxicity through antioxidant and anti-inflammatory actions.
MeSH Terms
-
Alanine Transaminase
Ammonia
Animals
Aspartate Aminotransferases
Bile Ducts
Carboxymethylcellulose Sodium
Catalase
Hepatic Encephalopathy*
Hydrogen Sulfide
Inflammation
Ligation
Liver
Liver Diseases
Malondialdehyde
Models, Animal
N-Methylaspartate
Necrosis
Peroxidase
Rats*
Silymarin
Sodium*
Alanine Transaminase
Ammonia
Aspartate Aminotransferases
Carboxymethylcellulose Sodium
Catalase
Hydrogen Sulfide
Malondialdehyde
N-Methylaspartate
Peroxidase
Silymarin
Sodium
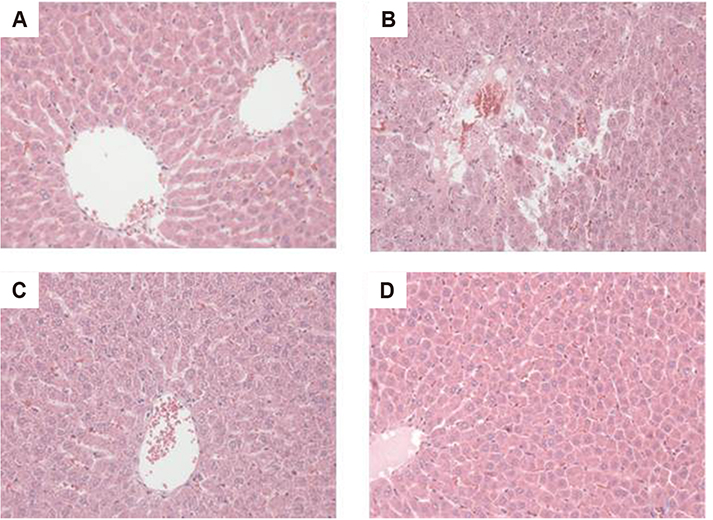
Figure
Reference
-
1. Gow AG. Hepatic encephalopathy. Vet Clin North Am Small Anim Pract. 2017; 47:585–599.
Article2. Gerber T, Schomerus H. Hepatic encephalopathy in liver cirrhosis: pathogenesis, diagnosis and management. Drugs. 2000; 60:1353–1370.3. Ong JP, Mullen KD. Hepatic encephalopathy. Eur J Gastroenterol Hepatol. 2001; 13:325–334.
Article4. Mas A. Hepatic encephalopathy: from pathophysiology to treatment. Digestion. 2006; 73:Suppl 1. 86–93.
Article5. Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002; 35:716–721.
Article6. Butterworth RF, Norenberg MD, Felipo V, Ferenci P, Albrecht J, Blei AT. Experimental models of hepatic encephalopathy: ISHEN guidelines. Liver Int. 2009; 29:783–788.
Article7. Kountouras J, Billing BH, Scheuer PJ. Prolonged bile duct obstruction: a new experimental model for cirrhosis in the rat. Br J Exp Pathol. 1984; 65:305–311.8. Greve JW, Gouma DJ, Soeters PB, Buurman WA. Suppression of cellular immunity in obstructive jaundice is caused by endotoxins: a study with germ-free rats. Gastroenterology. 1990; 98:478–485.
Article9. Rodrigo R, Jover R, Candela A, Compañ A, Sáez-Valero J, Erceg S, Felipo V. Bile duct ligation plus hyperammonemia in rats reproduces the alterations in the modulation of soluble guanylate cyclase by nitric oxide in brain of cirrhotic patients. Neuroscience. 2005; 130:435–443.
Article10. Huang YT, Hsu YC, Chen CJ, Liu CT, Wei YH. Oxidative-stress-related changes in the livers of bile-duct-ligated rats. J Biomed Sci. 2003; 10:170–178.
Article11. Tag CG, Sauer-Lehnen S, Weiskirchen S, Borkham-Kamphorst E, Tolba RH, Tacke F, Weiskirchen R. Bile duct ligation in mice: induction of inflammatory liver injury and fibrosis by obstructive cholestasis. J Vis Exp. 2015; (96):52438.
Article12. Holecek M. Ammonia and amino acid profiles in liver cirrhosis: effects of variables leading to hepatic encephalopathy. Nutrition. 2015; 31:14–20.
Article13. Giménez-Garzó C, Salhi D, Urios A, Ruíz-Sauri A, Carda C, Montoliu C, Felipo V. Rats with mild bile duct ligation show hepatic encephalopathy with cognitive and motor impairment in the absence of cirrhosis: effects of alcohol ingestion. Neurochem Res. 2015; 40:230–240.
Article14. Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993; 361:31–39.
Article15. Felipo V. Hepatic encephalopathy: effects of liver failure on brain function. Nat Rev Neurosci. 2013; 14:851–858.
Article16. Vieira MM, Schmidt J, Ferreira JS, She K, Oku S, Mele M, Santos AE, Duarte CB, Craig AM, Carvalho AL. Multiple domains in the C-terminus of NMDA receptor GluN2B subunit contribute to neuronal death following in vitro ischemia. Neurobiol Dis. 2016; 89:223–234.
Article17. Llansola M, Rodrigo R, Monfort P, Montoliu C, Kosenko E, Cauli O, Piedrafita B, El Mlili N, Felipo V. NMDA receptors in hyperammonemia and hepatic encephalopathy. Metab Brain Dis. 2007; 22:321–335.
Article18. Kim ST, Kyung EJ, Suh JS, Lee HS, Lee JH, Chae SI, Park ES, Chung YH, Bae J, Lee TJ, Lee WM, Sohn UD, Jeong JH. Phosphatidylcholine attenuated docetaxel-induced peripheral neurotoxicity in rats. Drug Chem Toxicol. 2018; 41:476–485.
Article19. Wang D, Jacobs SA, Tsien JZ. Targeting the NMDA receptor subunit NR2B for treating or preventing age-related memory decline. Expert Opin Ther Targets. 2014; 18:1121–1130.
Article20. Sun YR, Sun YG, Zhang Q, Wang XD, Wang X, Sun LJ. Learning and memory capacity and NMDA receptor expression in shen deficiency constitution rats. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2016; 36:597–601.21. Bhambhani Y, Singh M. Physiological effects of hydrogen sulfide inhalation during exercise in healthy men. J Appl Physiol (1985). 1991; 71:1872–1877.
Article22. Truong DH, Eghbal MA, Hindmarsh W, Roth SH, O'Brien PJ. Molecular mechanisms of hydrogen sulfide toxicity. Drug Metab Rev. 2006; 38:733–744.
Article23. Hildebrandt TM. Modulation of sulfide oxidation and toxicity in rat mitochondria by dehydroascorbic acid. Biochim Biophys Acta. 2011; 1807:1206–1213.
Article24. Calvert JW, Coetzee WA, Lefer DJ. Novel insights into hydrogen sulfide--mediated cytoprotection. Antioxid Redox Signal. 2010; 12:1203–1217.
Article25. Bos EM, Snijder PM, Jekel H, Weij M, Leemans JC, van Dijk MC, Hillebrands JL, Lisman T, van Goor H, Leuvenink HG. Beneficial effects of gaseous hydrogen sulfide in hepatic ischemia/reperfusion injury. Transpl Int. 2012; 25:897–908.
Article26. Zhang Q, Fu H, Zhang H, Xu F, Zou Z, Liu M, Wang Q, Miao M, Shi X. Hydrogen sulfide preconditioning protects rat liver against ischemia/reperfusion injury by activating Akt-GSK-3β signaling and inhibiting mitochondrial permeability transition. PLoS One. 2013; 8:e74422.
Article27. Tu FP, Li JX, Li Q, Wang J. Effects of hydrogen sulfide on cognitive dysfunction and NR2B in rats. J Surg Res. 2016; 205:426–431.
Article28. Ahmadi S, Poureidi M, Rostamzadeh J. Hepatic encephalopathy induces site-specific changes in gene expression of GluN1 subunit of NMDA receptor in rat brain. Metab Brain Dis. 2015; 30:1035–1041.
Article29. Singh S, Shackleton G, Ah-Sing E, Chakraborty J, Bailey ME. Antioxidant defenses in the bile duct-ligated rat. Gastroenterology. 1992; 103:1625–1629.
Article30. Vázquez-Gil MJ, Mesonero MJ, Flores O, Criado M, Hidalgo F, Arévalo MA, Sánchez-Rodríguez A, Tuñón MJ, López-Novoa JM, Esteller A. Sequential changes in redox status and nitric oxide synthases expression in the liver after bile duct ligation. Life Sci. 2004; 75:717–732.
Article31. Nam Y, Bae J, Jeong JH, Ko SK, Sohn UD. Protective effect of ultrasonication-processed ginseng berry extract on the D-galactosamine/lipopolysaccharide-induced liver injury model in rats. J Ginseng Res. 2018; 42:540–548.
Article32. Song Z, Deaciuc I, Song M, Lee DY, Liu Y, Ji X, McClain C. Silymarin protects against acute ethanol-induced hepatotoxicity in mice. Alcohol Clin Exp Res. 2006; 30:407–413.
Article33. Katayama K. Ammonia metabolism and hepatic encephalopathy. Hepatol Res. 2004; 30S:73–80.
Article34. Lockwood AH. Blood ammonia levels and hepatic encephalopathy. Metab Brain Dis. 2004; 19:345–349.
Article35. Butterworth RF, Giguère JF, Michaud J, Lavoie J, Layrargues GP. Ammonia: key factor in the pathogenesis of hepatic encephalopathy. Neurochem Pathol. 1987; 6:1–12.
Article36. Norenberg MD. Astrocytic-ammonia interactions in hepatic encephalopathy. Semin Liver Dis. 1996; 16:245–253.
Article37. Jang HS, Um SI, Lee SH, Whang WK, Min YS, Park SY, Sohn UD. The protective mechanism of QGC in feline esophageal epithelial cells by interleukin-1β treatment. Arch Pharm Res. 2017; 40:204–213.
Article38. Lee YJ, Kim SJ, Kwon KW, Lee WM, Im WJ, Sohn UD. Inhibitory effect of FSLLRY-NH2 on inflammatory responses induced by hydrogen peroxide in HepG2 cells. Arch Pharm Res. 2017; 40:854–863.
Article39. Plebani M, Panozzo MP, Basso D, De Paoli M, Biasin R, Infantolino D. Cytokines and the progression of liver damage in experimental bile duct ligation. Clin Exp Pharmacol Physiol. 1999; 26:358–363.
Article40. Fernández-Martínez E, Pérez-Alvarez V, Tsutsumi V, Shibayama M, Muriel P. Chronic bile duct obstruction induces changes in plasma and hepatic levels of cytokines and nitric oxide in the rat. Exp Toxicol Pathol. 2006; 58:49–58.
Article41. Kimura H. Hydrogen sulfide induces cyclic AMP and modulates the NMDA receptor. Biochem Biophys Res Commun. 2000; 267:129–133.
Article42. Sakamoto K, Suzuki Y, Kurauchi Y, Mori A, Nakahara T, Ishii K. Hydrogen sulfide attenuates NMDA-induced neuronal injury via its anti-oxidative activity in the rat retina. Exp Eye Res. 2014; 120:90–96.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hepatic encephalopathy
- Clinical observations of hepatic encephalopathy
- Effect of 4-Methylpyrazole for Acetaminophen Hepatotoxicity in a Rat Model
- Evaluation and Management of Hepatic Encephalopathy: Current Status and Future Directions
- A Case of Recurrent Hepatic Encephalopathy Secondary to Spontaneous Intrahepatic Portosystemic Venous Shunt