Yeungnam Univ J Med.
2019 May;36(2):105-108. 10.12701/yujm.2019.00108.
Impact of calcineurin inhibitors on rat glioma cells viability
- Affiliations
-
- 1Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea. mdjin922@gmail.com
- 2Keimyung University Kidney Institute, Keimyung University School of Medicine, Daegu, Korea.
- 3Department of Biochemistry, Keimyung University School of Medicine, Daegu, Korea.
- KMID: 2449322
- DOI: http://doi.org/10.12701/yujm.2019.00108
Abstract
- BACKGROUND
Although kidney transplantation outcomes have improved dramatically after using calcineurin inhibitors (CNIs), CNI toxicity continues to be reported and the mechanism remains uncertain. Here, we investigated the neurotoxicity of CNIs by focusing on the viability of glioma cells.
METHODS
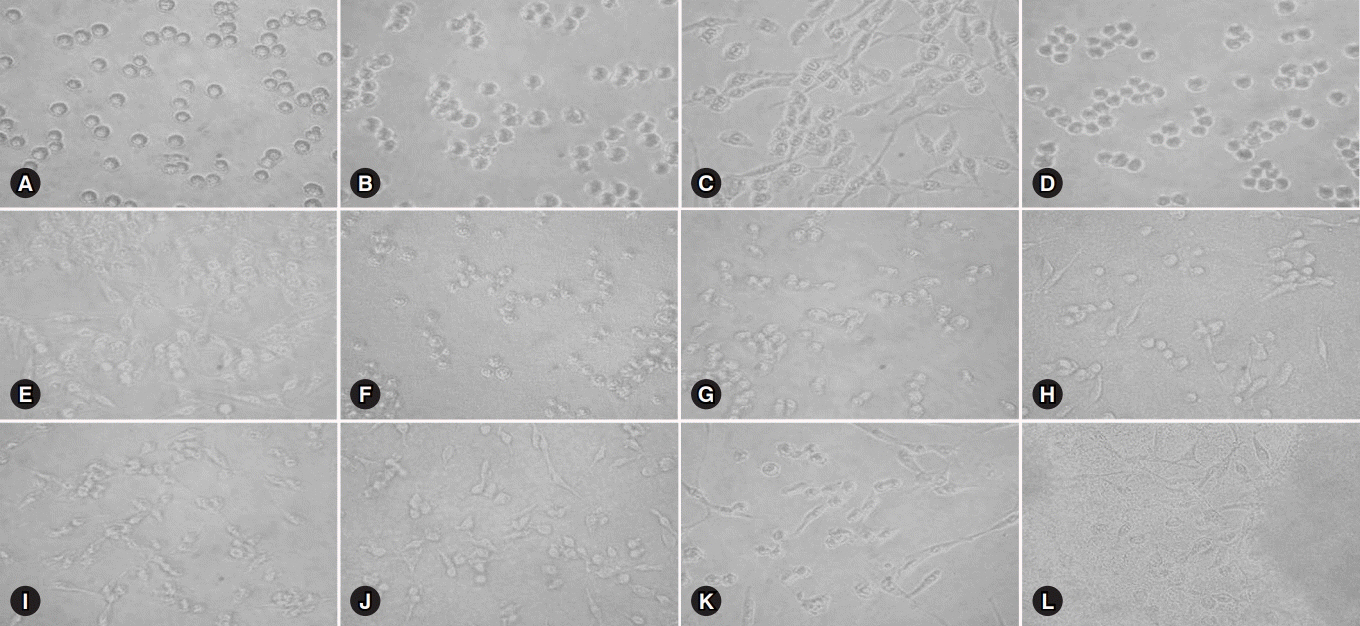
Glioma cells were treated with several concentrations of CNIs for 24 hours at 37℃ and their cell viability was evaluated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay.
RESULTS
Exposure to 0, 0.25, 0.5, 2.5, 5.0, and 10.0 mM concentrations respectively showed 100%, 64.3%, 61.3%, 68.1%, 62.4%, and 68.6% cell viability for cyclosporine and 100%, 38.6%, 40.8%, 43.7%, 37.8%, and 43.0% for tacrolimus. The direct toxic effect of tacrolimus on glioma cell viability was stronger than that of cyclosporine at the same concentration.
CONCLUSION
CNIs can cause neurological side effects by directly exerting cytotoxic effects on brain cells. Therefore, we should carefully monitor the neurologic symptoms and level of CNIs in kidney transplant patients.
MeSH Terms
Figure
Reference
-
References
1. Pascual M, Theruvath T, Kawai T, Tolkoff-Rubin N, Cosimi AB. Strategies to improve long-term outcomes after renal transplantation. N Engl J Med. 2002; 346:580–90.
Article2. Malvezzi P, Rostaing L. The safety of calcineurin inhibitors for kidney-transplant patients. Expert Opin Drug Saf. 2015; 14:1531–46.
Article3. Burdmann EA, Andoh TF, Yu L, Bennett WM. Cyclosporine nephrotoxicity. Semin Nephrol. 2003; 23:465–76.
Article4. Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009; 4:481–508.
Article5. Anghel D, Tanasescu R, Campeanu A, Lupescu I, Podda G, Bajenaru O. Neurotoxicity of immunosuppressive therapies in organ transplantation. Maedica (Buchar). 2013; 8:170–5.6. Senzolo M, Ferronato C, Burra P. Neurologic complications after solid organ transplantation. Transpl Int. 2009; 22:269–78.
Article7. Besenski N, Rumboldt Z, Emovon O, Nicholas J, Kini S, Milutinovic J, et al. Brain MR imaging abnormalities in kidney transplant recipients. AJNR Am J Neuroradiol. 2005; 26:2282–9.8. Coley SC, Porter DA, Calamante F, Chong WK, Connelly A. Quantitative MR diffusion mapping and cyclosporine-induced neurotoxicity. AJNR Am J Neuroradiol. 1999; 20:1507–10.9. Schwartz RB, Bravo SM, Klufas RA, Hsu L, Barnes PD, Robson CD, et al. Cyclosporine neurotoxicity and its relationship to hypertensive encephalopathy: CT and MR findings in 16 cases. AJR Am J Roentgenol. 1995; 165:627–31.
Article10. Oh YL, Han SY, Mun KH, Choi HJ, Kim HY, Hwang EA, et al. Cyclosporine-induced apoptosis in osteosarcoma cells. Transplant Proc. 2006; 38:2237–9.
Article11. Han SY, Chang EJ, Choi HJ, Nam SI, Lee NH, Kwak CS, et al. Total antioxidant status and oxygen free radicals during hepatic regeneration. Transplant Proc. 2006; 38:2214–5.
Article12. Hoorn EJ, Walsh SB, McCormick JA, Zietse R, Unwin RJ, Ellison DH. Pathogenesis of calcineurin inhibitor-induced hypertension. J Nephrol. 2012; 25:269–75.
Article13. Chakkera HA, Mandarino LJ. Calcineurin inhibition and new-onset diabetes mellitus after transplantation. Transplantation. 2013; 95:647–52.
Article14. Rosendal F, Bjarkam CR, Larsen M, Hansen HE, Madsen M, Sørensen JC, et al. Does chronic low-dose treatment with cyclosporine influence the brain? A histopathological study in pigs. Transplant Proc. 2005; 37:3305–8.15. Kiemeneij IM, de Leeuw FE, Ramos LM, van Gijn J. Acute headache as a presenting symptom of tacrolimus encephalopathy. J Neurol Neurosurg Psychiatry. 2003; 74:1126–7.
Article16. Thompson CB, June CH, Sullivan KM, Thomas ED. Association between cyclosporin neurotoxicity and hypomagnesaemia. Lancet. 1984; 2:1116–20.
Article17. Gijtenbeek JM, van den Bent MJ, Vecht CJ. Cyclosporine neurotoxicity: a review. J Neurol. 1999; 246:339–46.
Article18. Kou R, Greif D, Michel T. Dephosphorylation of endothelial nitric-oxide synthase by vascular endothelial growth factor. Implications for the vascular responses to cyclosporin A. J Biol Chem. 2002; 277:29669–73.19. Han SY, Mun KC, Choi HJ, Kwak CS, Bae JH, Suh SI, et al. Effects of cyclosporine and tacrolimus on the oxidative stress in cultured mesangial cells. Transplant Proc. 2006; 38:2240–1.
Article20. Jang YH, Lee YC, Park NH, Shin HY, Mun KC, Choi MS, et al. Polyphenol (-)-epigallocatechin gallate protection from ischemia/reperfusion-induced renal injury in normotensive and hypertensive rats. Transplant Proc. 2006; 38:2190–4.
Article21. Folbergrová J, Li PA, Uchino H, Smith ML, Siesjö BK. Changes in the bioenergetic state of rat hippocampus during 2.5 min of ischemia, and prevention of cell damage by cyclosporin A in hyperglycemic subjects. Exp Brain Res. 1997; 114:44–50.
Article22. Beal MF. Mitochondrial dysfunction in neurodegenerative diseases. Biochim Biophys Acta. 1998; 1366:211–23.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Implications of Calcineurin/NFAT Inhibitors' Regulation of Dendritic Cells and Innate Immune Cells in Islet Xenotransplantation
- The Efficacy of Topical Calcineurin Inhibitors for Treating Lichen Striatus
- Mechanisms and clinical applications of immunosuppressive medications
- Regulation of the Expression of a Calcineurin Inhibitor, ZAKI-4alpha
- Treatment of Atopic Dermatitis: an Update and Review of the Literature